APP Function and Lipids: A Bidirectional Link
- PMID: 28344547
- PMCID: PMC5344993
- DOI: 10.3389/fnmol.2017.00063
APP Function and Lipids: A Bidirectional Link
Abstract
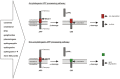
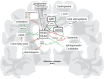
Extracellular neuritic plaques, composed of aggregated amyloid-β (Aβ) peptides, are one of the major histopathological hallmarks of Alzheimer's disease (AD), a progressive, irreversible neurodegenerative disorder and the most common cause of dementia in the elderly. One of the most prominent risk factor for sporadic AD, carrying one or two aberrant copies of the apolipoprotein E (ApoE) ε4 alleles, closely links AD to lipids. Further, several lipid classes and fatty acids have been reported to be changed in the brain of AD-affected individuals. Interestingly, the observed lipid changes in the brain seem not only to be a consequence of the disease but also modulate Aβ generation. In line with these observations, protective lipids being able to decrease Aβ generation and also potential negative lipids in respect to AD were identified. Mechanistically, Aβ peptides are generated by sequential proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretase. The α-secretase appears to compete with β-secretase for the initial cleavage of APP, preventing Aβ production. All APP-cleaving secretases as well as APP are transmembrane proteins, further illustrating the impact of lipids on Aβ generation. Beside the pathological impact of Aβ, accumulating evidence suggests that Aβ and the APP intracellular domain (AICD) play an important role in regulating lipid homeostasis, either by direct effects or by affecting gene expression or protein stability of enzymes involved in the de novo synthesis of different lipid classes. This review summarizes the current literature addressing the complex bidirectional link between lipids and AD and APP processing including lipid alterations found in AD post mortem brains, lipids that alter APP processing and the physiological functions of Aβ and AICD in the regulation of several lipid metabolism pathways.
Keywords: AICD; APP processing; Abeta cholesterol; PUFA; gangliosides; lipids; sphingolipids; sulfatides.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

