Drug Repurposing of the Anthelmintic Niclosamide to Treat Multidrug-Resistant Leukemia
- PMID: 28344555
- PMCID: PMC5344920
- DOI: 10.3389/fphar.2017.00110
Drug Repurposing of the Anthelmintic Niclosamide to Treat Multidrug-Resistant Leukemia
Abstract
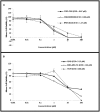
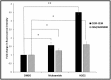
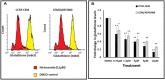
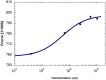
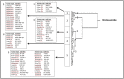
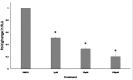
Multidrug resistance, a major problem that leads to failure of anticancer chemotherapy, requires the development of new drugs. Repurposing of established drugs is a promising approach for overcoming this problem. An example of such drugs is niclosamide, a known anthelmintic that is now known to be cytotoxic and cytostatic against cancer cells. In this study, niclosamide showed varying activity against different cancer cell lines. It revealed better activity against hematological cancer cell lines CCRF-CEM, CEM/ADR5000, and RPMI-8226 compared to the solid tumor cell lines MDA-MB-231, A549, and HT-29. The multidrug resistant CEM/ADR5000 cells were similar sensitive as their sensitive counterpart CCRF-CEM (resistance ration: 1.24). Furthermore, niclosamide caused elevations in reactive oxygen species and glutathione (GSH) levels in leukemia cells. GSH synthetase (GS) was predicted as a target of niclosamide. Molecular docking showed that niclosamide probably binds to the ATP-binding site of GS with a binding energy of -9.40 kcal/mol. Using microscale thermophoresis, the binding affinity between niclosamide and recombinant human GS was measured (binding constant: 5.64 μM). COMPARE analyses of the NCI microarray database for 60 cell lines showed that several genes, including those involved in lipid metabolism, correlated with cellular responsiveness to niclosamide. Hierarchical cluster analysis showed five major branches with significant differences between sensitive and resistant cell lines (p = 8.66 × 105). Niclosamide significantly decreased nuclear factor of activated T-cells (NFAT) activity as predicted by promoter binding motif analysis. In conclusion, niclosamide was more active against hematological malignancies compared to solid tumors. The drug was particularly active against the multidrug-resistant CEM/ADR5000 leukemia cells. Inhibition of GSH synthesis and NFAT signaling were identified as relevant mechanisms for the anticancer activity of niclosamide. Gene expression profiling predicted the sensitivity or resistance of cancer cells to niclosamide.
Keywords: chemotherapy; drug resistance; oxidative stress; pharmacogenomics; transcription factors.
Figures

References
-
- Allen J. D., Brinkhuis R. F., van Deemter L., Wijnholds J., Schinkel A. H. (2000). Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res. 60 5761–5766. - PubMed
-
- Bartsevich V. V., Juliano R. L. (2000). Regulation of the MDR1 gene by transcriptional repressors selected using peptide combinatorial libraries. Mol. Pharmacol. 58 1–10. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

