Comparative insights into the saccharification potentials of a relatively unexplored but robust Penicillium funiculosum glycoside hydrolase 7 cellobiohydrolase
- PMID: 28344646
- PMCID: PMC5360062
- DOI: 10.1186/s13068-017-0752-x
Comparative insights into the saccharification potentials of a relatively unexplored but robust Penicillium funiculosum glycoside hydrolase 7 cellobiohydrolase
Abstract
Background: GH7 cellobiohydrolases (CBH1) are vital for the breakdown of cellulose. We had previously observed the enzyme as the most dominant protein in the active cellulose-hydrolyzing secretome of the hypercellulolytic ascomycete-Penicillium funiculosum (NCIM1228). To understand its contributions to cellulosic biomass saccharification in comparison with GH7 cellobiohydrolase from the industrial workhorse-Trichoderma reesei, we natively purified and functionally characterized the only GH7 cellobiohydrolase identified and present in the genome of the fungus.
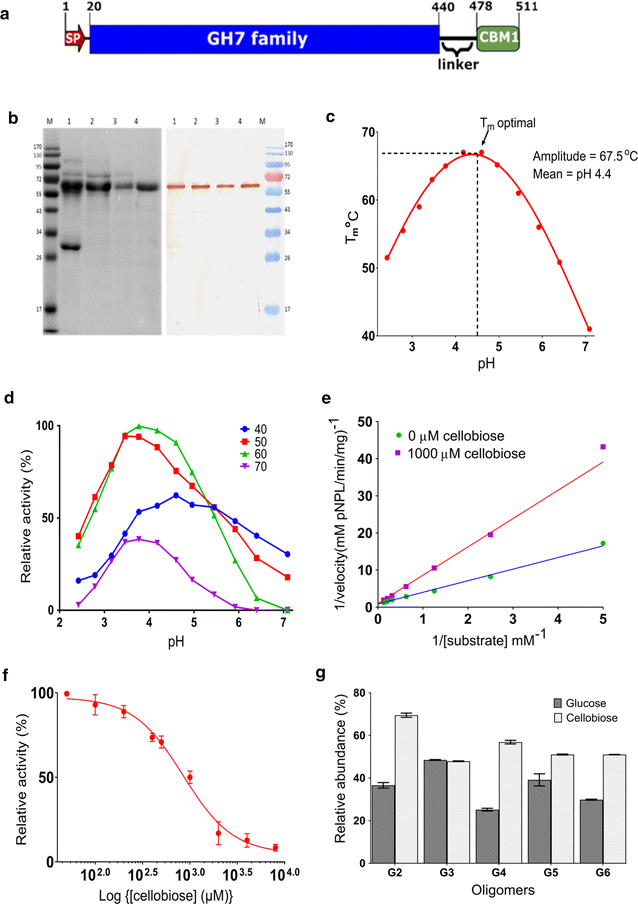
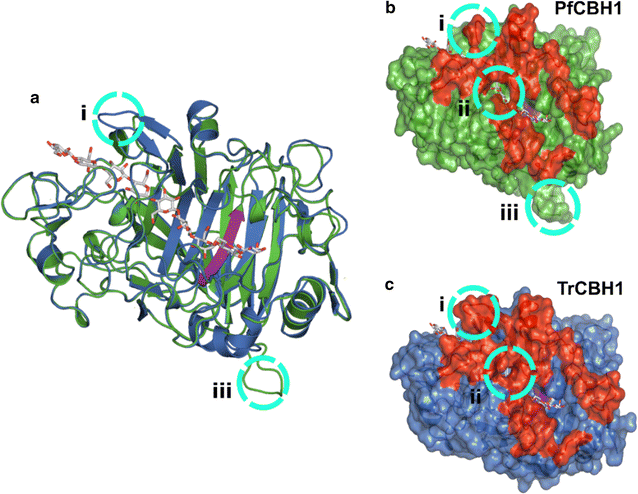
Results: There were marginal differences observed in the stability of both enzymes, with P. funiculosum (PfCBH1) showing an optimal thermal midpoint (Tm) of 68 °C at pH 4.4 as against an optimal Tm of 65 °C at pH 4.7 for T. reesei (TrCBH1). Nevertheless, PfCBH1 had an approximate threefold lower binding affinity (Km), an 18-fold higher turnover rate (kcat), a sixfold higher catalytic efficiency as well as a 26-fold higher enzyme-inhibitor complex equilibrium dissociation constant (Ki) than TrCBH1 on p-nitrophenyl-β-d-lactopyranoside (pNPL). Although both enzymes hydrolyzed cellooligomers (G2-G6) and microcrystalline cellulose, releasing cellobiose and glucose as the major products, the propensity was more with PfCBH1. We equally observed this trend during the hydrolysis of pretreated wheat straws in tandem with other core cellulases under the same conditions. Molecular dynamic simulations conducted on a homology model built using the TrCBH1 structure (PDB ID: 8CEL) as a template enabled us to directly examine the effects of substrate and products on the protein dynamics. While the catalytic triads-EXDXXE motifs-were conserved between the two enzymes, subtle variations in regions enclosing the catalytic path were observed, and relations to functionality highlighted.
Conclusion: To the best of our knowledge, this is the first report about a comprehensive and comparative description of CBH1 from hypercellulolytic ascomycete-P. funiculosum NCIM1228, against the backdrop of the same enzyme from the industrial workhorse-T. reesei. Our study reveals PfCBH1 as a viable alternative for CBH1 from T. reesei in industrial cellulase cocktails.
Keywords: Biomass degradation; CAZymes: glycoside hydrolases: Penicillium funiculosum; Cellobiohydrolase 1; Molecular dynamics.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

