Synthesis, characterization and toxicity studies of pyridinecarboxaldehydes and L-tryptophan derived Schiff bases and corresponding copper (II) complexes
- PMID: 28344771
- PMCID: PMC5333612
- DOI: 10.12688/f1000research.9226.1
Synthesis, characterization and toxicity studies of pyridinecarboxaldehydes and L-tryptophan derived Schiff bases and corresponding copper (II) complexes
Abstract
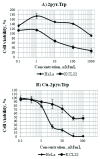
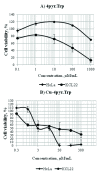
Schiff bases and their metal-complexes are versatile compounds exhibiting a broad range of biological activities and thus actively used in the drug development process. The aim of the present study was the synthesis and characterization of new Schiff bases and their copper (II) complexes, derived from L-tryptophan and isomeric (2-; 3-; 4-) pyridinecarboxaldehydes, as well as the assessment of their toxicity in vitro. The optimal conditions of the Schiff base synthesis resulting in up to 75-85% yield of target products were identified. The structure-activity relationship analysis indicated that the location of the carboxaldehyde group at 2-, 3- or 4-position with regard to nitrogen of the pyridine ring in aldehyde component of the L-tryptophan derivative Schiff bases and corresponding copper complexes essentially change the biological activity of the compounds. The carboxaldehyde group at 2- and 4-positions leads to the higher cytotoxic activity, than that of at 3-position, and the presence of the copper in the complexes increases the cytotoxicity. Based on toxicity classification data, the compounds with non-toxic profile were identified, which can be used as new entities in the drug development process using Schiff base scaffold.
Keywords: HeLa; KCL-22; L-tryptophan; Schiff base; copper (II) complex; cytotoxicity; synthesis.
Conflict of interest statement
Competing interests: No competing interests were disclosed.
Figures




References
-
- Zoubi WAl: Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works. Int J Org Chem. 2013;3:73–95. 10.4236/ijoc.2013.33A008 - DOI
-
- Arulmurugan S, Kavitha HP, Venkatraman BR: Biological activities of Schiff base and its complexes: A review. Rasayan J Chem. 2010;3(3):385–410. Reference Source
-
- Kajal A, Bala S, Kamboj S, et al. : Schiff Bases: A Versatile Pharmacophore. J Catal. 2013;2013:1–14. 10.1155/2013/893512 - DOI
-
- Paul P: Ruthenium, osmium and rhodium complexes of polypyridyl ligands: Metal-promoted activities, stereochemical aspects and electrochemical properties. J Chem Sci. 2002;114(4):269–276. 10.1007/BF02703819 - DOI
-
- Cimerman Z, Miljanić S, Galić N: Schiff Bases Derived from Aminopyridines as Spectrofluorimetric Analytical Reagents. Croat Chem Acta. 2000;73(1):81–95. Reference Source
LinkOut - more resources
Full Text Sources
Other Literature Sources

