CD13hi Neutrophil-like myeloid-derived suppressor cells exert immune suppression through Arginase 1 expression in pancreatic ductal adenocarcinoma
- PMID: 28344866
- PMCID: PMC5353902
- DOI: 10.1080/2162402X.2016.1258504
CD13hi Neutrophil-like myeloid-derived suppressor cells exert immune suppression through Arginase 1 expression in pancreatic ductal adenocarcinoma
Abstract
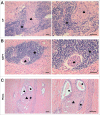
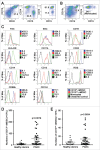
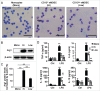
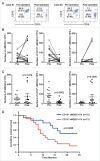
Perineural invasion and immunosuppressive tumor microenvironment are the distinct features of pancreatic ductal adenocarcinoma (PDAC). Heterogeneous myeloid-derived suppressor cells (MDSCs) are potent suppressors of antitumor immunity, posing obstacles for cancer immunotherapy. Increasing evidences have demonstrated the accumulation of MDSCs in PDAC patients. However, the role of MDSCs in perineural invasion of PDAC and the existence of novel MDSC subsets during PDAC remain unclear. This study found that lymphocytic perineural cuffs were frequently present in chronic pancreatitis (CP) tissues and adjacent non-neoplastic pancreatic tissues (ANPTs), but not in PDAC with perineural invasion. Meanwhile, we found that neutrophil-like MDSCs (nMDSCs), but not monocyte-like MDSCs (mMDSCs), were significantly increased in PBMCs and tumor tissues of PDAC patients. Further observation identified two distinct subsets of nMDSCs, CD13hi and CD13low nMDSCs in PDAC patients, which have not been reported previously. Despite a similar morphology, CD13hi nMDSCs expressed higher levels of CD11b, CD33, CD16 and arginase 1 but lower levels of CD66b than CD13low nMDSCs. Importantly, CD13hi MDSCs, compared with CD13low nMDSCs, more effectively suppressed alloreactive T cell responses via an arginase-1-related mechanism. After tumor resection, the circulating CD13hi nMDSCs were decreased markedly. PDAC patients with more CD13hi nMDSCs had a shorter overall survival than those with less CD13hi nMDSCs. To conclude, we identified two novel MDSC subsets with different characteristics and functions in PDAC, demonstrated the association of the two MDSC subsets with cancer progression, and explored their roles in perineural invasion and immune escape of PDAC.
Keywords: Immune escape; myeloid-derived suppressor cells; pancreatic ductal adenocarcinoma; tumor microenvironment.
Figures



References
-
- Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol 2013; 91:493-502; PMID:23797066; http://dx.doi.org/10.1038/icb.2013.29 - DOI - PubMed
-
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175 - DOI - PMC - PubMed
-
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:19342621; http://dx.doi.org/10.4049/jimmunol.0802740 - DOI - PMC - PubMed
-
- Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014; 1319:47-65; PMID:24965257; http://dx.doi.org/10.1111/nyas.12469 - DOI - PubMed
-
- Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol 2011; 11:802-7; PMID:21237299; http://dx.doi.org/10.1016/j.intimp.2011.01.003 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
