Grape seed proanthocyanidin extract protects lymphocytes against histone-induced apoptosis
- PMID: 28344907
- PMCID: PMC5363264
- DOI: 10.7717/peerj.3108
Grape seed proanthocyanidin extract protects lymphocytes against histone-induced apoptosis
Abstract
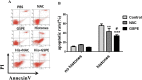
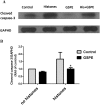
Apoptosis of lymphocytes is associated with immunosuppression and poor prognosis in sepsis. Our previous report showed that histones, nuclear proteins released from damaged or dying cells in sepsis, can mediate lymphocyte apoptosis via mitochondria damage. Grape seed proanthocyanidin extract (GSPE), a natural substance with protective properties against oxidative stress, plays a vital role in cell and mitochondria protection. We thus hypothesized that GSPE may play a protective role in histone-induced lymphocyte apoptosis through its anti-oxidative properties. In this study, we investigated the protective efficacy of GSPE on lymphocyte apoptosis induced by extracellular histones, a main contributor of death in sepsis. Human blood lymphocytes were treated with 50 μg/ml histones, 2 μg/ml GSPE, or a combination of both. A total of 100 μM N-acetylcysteine (NAC), a reactive oxygen species (ROS) inhibitor, was used as a positive control for GSPE. Apoptosis, intracellular ROS levels, mitochondrial membrane potential, Bcl-2 expression, and caspase-3 cleavage were measured. Our data clearly indicate that GSPE significantly inhibited lymphocyte apoptosis, generation of ROS, the loss of mitochondrial membrane potential, the decrease in Bcl-2 expression, and caspase-3 activation induced by extracellular histones. In conclusion, we show that GSPE has a protective effect on lymphocyte apoptosis induced by extracellular histones. This study suggests GSPE as a potential therapeutic agent that could help reduce lymphocyte apoptosis, and thus the state of immunosuppression was observed in septic patients.
Keywords: Grape seed proanthocyanidin extract; Histones; Lymphocyte apoptosis; Mitochondrial injury; Reactive oxygen species.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Ahn SH, Kim HJ, Jeong I, Hong YJ, Kim MJ, Rhie DJ, Jo YH, Hahn SJ, Yoon SH. Grape seed proanthocyanidin extract inhibits glutamate-induced cell death through inhibition of calcium signals and nitric oxide formation in cultured rat hippocampal neurons. BMC Neuroscience. 2011;12(1):78. doi: 10.1186/1471-2202-12-78. - DOI - PMC - PubMed
-
- Demirkaya E, Avci A, Kesik V, Karslioglu Y, Oztas E, Kismet E, Gokcay E, Durak I, Koseoglu V. Cardioprotective roles of aged garlic extract, grape seed proanthocyanidin, and hazelnut on doxorubicin-induced cardiotoxicity. Canadian Journal of Physiology and Pharmacology. 2009;87(8):633–640. doi: 10.1139/y09-051. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

