Global Manufacturing of CAR T Cell Therapy
- PMID: 28344995
- PMCID: PMC5363291
- DOI: 10.1016/j.omtm.2016.12.006
Global Manufacturing of CAR T Cell Therapy
Abstract
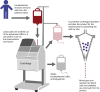
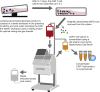
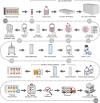
Immunotherapy using chimeric antigen receptor-modified T cells has demonstrated high response rates in patients with B cell malignancies, and chimeric antigen receptor T cell therapy is now being investigated in several hematologic and solid tumor types. Chimeric antigen receptor T cells are generated by removing T cells from a patient's blood and engineering the cells to express the chimeric antigen receptor, which reprograms the T cells to target tumor cells. As chimeric antigen receptor T cell therapy moves into later-phase clinical trials and becomes an option for more patients, compliance of the chimeric antigen receptor T cell manufacturing process with global regulatory requirements becomes a topic for extensive discussion. Additionally, the challenges of taking a chimeric antigen receptor T cell manufacturing process from a single institution to a large-scale multi-site manufacturing center must be addressed. We have anticipated such concerns in our experience with the CD19 chimeric antigen receptor T cell therapy CTL019. In this review, we discuss steps involved in the cell processing of the technology, including the use of an optimal vector for consistent cell processing, along with addressing the challenges of expanding chimeric antigen receptor T cell therapy to a global patient population.
Keywords: T lymphocytes; chimeric antigen receptor; global regulatory environment; lentiviral vector; manufacturing.
Figures

References
-
- Kuwana Y., Asakura Y., Utsunomiya N., Nakanishi M., Arata Y., Itoh S., Nagase F., Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987;149:960–968. - PubMed
-
- Finney H.M., Lawson A.D., Bebbington C.R., Weir A.N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998;161:2791–2797. - PubMed
-
- Maher J., Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat. Biotechnol. 2002;20:70–75. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

