OvAd1, a Novel, Potent, and Selective Chimeric Oncolytic Virus Developed for Ovarian Cancer by 3D-Directed Evolution
- PMID: 28345024
- PMCID: PMC5363728
- DOI: 10.1016/j.omto.2016.12.001
OvAd1, a Novel, Potent, and Selective Chimeric Oncolytic Virus Developed for Ovarian Cancer by 3D-Directed Evolution
Abstract
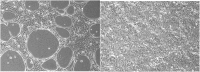
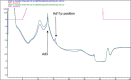
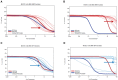
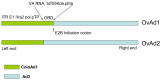
Effective therapeutics for ovarian cancer continue to be urgently needed, particularly for chemotherapy-resistant cases. Here we present both a 3D-Matrigel culture-based expansion of our directed evolution method for generation of oncolytic virotherapies and two promising ovarian-cancer targeted oncolytic viruses, OvAd1 and OvAd2. OvAd1 was developed using Matrigel cell cultures, whereas OvAd2 was developed in parallel using traditional monolayer tissue culture methods. Both viruses are potent against a panel of platinum-resistant ovarian cancer cell lines and are attenuated on normal cells in vitro, resulting in therapeutic windows of ∼200-fold. We observed two benefits of the use of Matrigel-based cultures for directed evolution of these oncolytics: (1) use of Matrigel generated a bioselected pool that was more strongly attenuated on normal cells while retaining its potency against ovarian cancer cells, and (2) in an ovarian carcinomatosis model, the Matrigel-derived virus OvAd1 suppressed all tumor growth while the non-Matrigel-derived virus was 50% effective. Neither virus stimulated formation of peritoneal adhesions as seen for Ad5-based therapies. Consequently, these viruses are novel candidates for development as new effective treatments for aggressive ovarian cancer.
Keywords: 3D cell culture; Matrigel; adenovirus; directed evolution; oncolytic; ovarian cancer; replicating; virotherapy.
Figures

References
-
- Lowe K.A., Chia V.M., Taylor A., O’Malley C., Kelsh M., Mohamed M., Mowat F.S., Goff B. An international assessment of ovarian cancer incidence and mortality. Gynecol. Oncol. 2013;130:107–114. - PubMed
-
- English D.P., Menderes G., Black J., Schwab C.L., Santin A.D. Molecular diagnosis and molecular profiling to detect treatment-resistant ovarian cancer. Expert Rev. Mol. Diagn. 2016;16:769–782. - PubMed
-
- Buller R.E., Runnebaum I.B., Karlan B.Y., Horowitz J.A., Shahin M., Buekers T., Petrauskas S., Kreienberg R., Slamon D., Pegram M. A phase I/II trial of rAd/p53 (SCH 58500) gene replacement in recurrent ovarian cancer. Cancer Gene Ther. 2002;9:553–566. - PubMed
-
- Aghi M., Martuza R.L. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. - PubMed
-
- Vasey P.A., Shulman L.N., Campos S., Davis J., Gore M., Johnston S., Kirn D.H., O’Neill V., Siddiqui N., Seiden M.V., Kaye S.B. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J. Clin. Oncol. 2002;20:1562–1569. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

