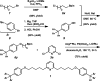
Exhaustive Suzuki-Miyaura reactions of polyhalogenated heteroarenes with alkyl boronic pinacol esters
- PMID: 28345090
- PMCID: PMC5491353
- DOI: 10.1039/c7cc00997f
Exhaustive Suzuki-Miyaura reactions of polyhalogenated heteroarenes with alkyl boronic pinacol esters
Abstract
A novel Suzuki-Miyaura protocol is described that enables the exhaustive alkylation of polychlorinated pyridines. This method facilitates a formal synthesis of normuscopyridine and the rapid assembly of a dumbbell shaped portion of a [2]rotaxane.
Figures
Similar articles
-
Selective and Serial Suzuki-Miyaura Reactions of Polychlorinated Aromatics with Alkyl Pinacol Boronic Esters.Org Lett. 2016 Sep 2;18(17):4440-3. doi: 10.1021/acs.orglett.6b02323. Epub 2016 Aug 18. Org Lett. 2016. PMID: 27537216 Free PMC article.
-
Speciation control during Suzuki-Miyaura cross-coupling of haloaryl and haloalkenyl MIDA boronic esters.Chemistry. 2015 Jun 8;21(24):8951-64. doi: 10.1002/chem.201500970. Epub 2015 May 8. Chemistry. 2015. PMID: 25959852
-
A C-H borylation approach to Suzuki-Miyaura coupling of typically unstable 2-heteroaryl and polyfluorophenyl boronates.Org Lett. 2012 Aug 17;14(16):4266-9. doi: 10.1021/ol301570t. Epub 2012 Aug 2. Org Lett. 2012. PMID: 22853624
-
Rapid, Homogenous, B-Alkyl Suzuki-Miyaura Cross-Coupling of Boronic Esters.J Org Chem. 2024 Nov 15;89(22):16195-16202. doi: 10.1021/acs.joc.4c00089. Epub 2024 Mar 14. J Org Chem. 2024. PMID: 38483187 Free PMC article.
-
Homologation and alkylation of boronic esters and boranes by 1,2-metallate rearrangement of boronate complexes.Chem Rec. 2009;9(1):24-39. doi: 10.1002/tcr.20168. Chem Rec. 2009. PMID: 19243084 Review.
Cited by
-
Photochemically Mediated Nickel-Catalyzed Synthesis of N-(Hetero)aryl Sulfamides.J Org Chem. 2020 May 15;85(10):6380-6391. doi: 10.1021/acs.joc.0c00139. Epub 2020 May 4. J Org Chem. 2020. PMID: 32312047 Free PMC article.
-
Photochemically-Mediated, Nickel-Catalyzed Synthesis of N-(Hetero)aryl Sulfamate Esters.Org Lett. 2019 Sep 6;21(17):7049-7054. doi: 10.1021/acs.orglett.9b02621. Epub 2019 Aug 22. Org Lett. 2019. PMID: 31436104 Free PMC article.
-
Directed Photochemically Mediated Nickel-Catalyzed (Hetero)arylation of Aliphatic C-H Bonds.J Am Chem Soc. 2023 Feb 13:10.1021/jacs.2c13409. doi: 10.1021/jacs.2c13409. Online ahead of print. J Am Chem Soc. 2023. PMID: 36780585 Free PMC article.
References
-
- Schinz H, Ruzicka L, Geyer U, Prelog V. Helv Chim Acta. 1946;29:1524.
- Fürstner A, Leitner A. Angew Chem Int Ed. 2003;42:308. - PubMed
- Davis JM, Truong A, Hamilton AD. Org Lett. 2005;7:5405. - PubMed
- Goldup SM, Leigh DA, Lusby PJ, McBurney RT, Slawin AMZ. Angew Chem Int Ed. 2008;47:6999. - PubMed
- Zhang YH, Shi BF, Yu JQ. J Am Chem Soc. 2009;131:5072. - PMC - PubMed
- Goetz AE, Garg NK. Nature Chemistry. 2013;5:54. - PMC - PubMed
-
-
For select C–H activation processes, see:
- Lewis JC, Bergman RG, Ellman JA. J Am Chem Soc. 2007;129:5332. - PMC - PubMed
- Nakao Y, Yamada Y, Kashihara N, Hiyama T. J Am Chem Soc. 2010;132:13666. - PubMed
-
For select examples that involve distinct bond-forming and deprotonation steps, see:
- Jeffrey JL, Sarpong R. Org Lett. 2012;14:5400. - PMC - PubMed
- Chen Q, León T, Knochel P. Angew Chem Int Ed. 2014;53:8746. - PubMed
- Hyodo I, Tobisu M, Chatani N. Chem Asian J. 2012;7:1357. - PubMed
-
For select Minisci type-pathways, see:
- Jin J, MacMillan DWC. Nature. 2015;525:87. - PMC - PubMed
- Gianatassio R, Kawamura S, Eprile CL, Foo K, Ge J, Burns AC, Collins MR, Baran PS. Angew Chem Int Ed. 2014;53:9851. - PMC - PubMed
- O’Hara F, Blackmond DG, Baran PS. J Am Chem Soc. 2013;135:12122. - PMC - PubMed
-
-
-
For select 2-pyridyl zinc reagents, see:
- Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan CM, Gianatassio R, Schmidt M, Eastgate MD, Baran PS. J Am Chem Soc. 2016;138:2174. - PMC - PubMed
- Colombe JR, Bernhardt S, Stathakis C, Buchwald SL, Knochel P. Org Lett. 2013;15:5754. - PMC - PubMed
- Luzung MR, Patel JS, Yin J. J Org Chem. 2010;75:8330. - PubMed
-
For select 2-pyridyl boron reagents, see:
- Dick GR, Woerly EM, Burke MD. Angew Chem Int Ed. 2012;51:2667. - PMC - PubMed
- Sakashita S, Takisawa M, Sugai J, Ito H, Yamamoto Y. Org Lett. 2013;15:4308. - PubMed
- Billingsley KL, Buchwald SL. Angew Chem Int Ed. 2008;47:4695. - PMC - PubMed
-
For 2-pyridyl tin reagents, see:
- Littke AF, Schawarz L, Fu GC. J Am Chem Soc. 2002;124:6343. - PubMed
- Bailey TR. Tetrahedron Lett. 1986;27:4407.
-
For 2-pyridyl aluminum reagents, see:
- Chen X, Zhou L, Li Y, Xie T, Zhou S. J Org Chem. 2014;79:230. - PubMed
-
-
-
For a select coupling reaction, see:
- Llaveria J, Leonori D, Aggarwal VK. J Am Chem Soc. 2015;137:10958. - PubMed
-
-
-
For select Negishi and Kumada reactions, see:
- Malhotra S, Seng PS, Koenig SG, Deese AJ, Ford KA. Org Lett. 2013;15:3698. - PubMed
- Fürstner A, Leitner A. Angew Chem Int Ed. 2003;42:308. - PubMed
- Getmanenko YA, Tweig RJ. J Org Chem. 2008;73:830. - PubMed
-
For cross-electrophile reactions, see:
- Everson DA, Buonomo JA, Weix DJ. Synlett. 2014;25:233. - PMC - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources