Pharmacogenomics of off-target adverse drug reactions
- PMID: 28345177
- PMCID: PMC5555876
- DOI: 10.1111/bcp.13294
Pharmacogenomics of off-target adverse drug reactions
Abstract
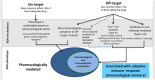
Off-target adverse drug reactions (ADRs) are associated with significant morbidity and costs to the healthcare system, and their occurrence is not predictable based on the known pharmacological action of the drug's therapeutic effect. Off-target ADRs may or may not be associated with immunological memory, although they can manifest with a variety of shared clinical features, including maculopapular exanthema, severe cutaneous adverse reactions (SCARs), angioedema, pruritus and bronchospasm. Discovery of specific genes associated with a particular ADR phenotype is a foundational component of clinical translation into screening programmes for their prevention. In this review, genetic associations of off-target drug-induced ADRs that have a clinical phenotype suggestive of an immunologically mediated process and their mechanisms are highlighted. A significant proportion of these reactions lack immunological memory and current data are informative for these ADRs with regard to disease pathophysiology, therapeutic targets and biomarkers which may identify patients at greatest risk. Although many serious delayed immune-mediated (IM)-ADRs show strong human leukocyte antigen associations, only a small subset have successfully been implemented in screening programmes. More recently, other factors, such as drug metabolism, have been shown to contribute to the risk of the IM-ADR. In the future, pharmacogenomic targets and an understanding of how they interact with drugs to cause ADRs will be applied to drug design and preclinical testing, and this will allow selection of optimal therapy to improve patient safety.
Keywords: abacavir; adverse drug reaction; aspirin-exacerbated respiratory disease; carbamazepine; human leukocyte antigen; pharmacogenomics.
© 2017 The British Pharmacological Society.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

