Early loss of subchondral bone following microfracture is counteracted by bone marrow aspirate in a translational model of osteochondral repair
- PMID: 28345610
- PMCID: PMC5366926
- DOI: 10.1038/srep45189
Early loss of subchondral bone following microfracture is counteracted by bone marrow aspirate in a translational model of osteochondral repair
Abstract
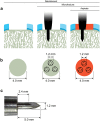
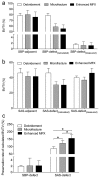
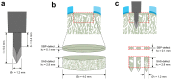
Microfracture of cartilage defects may induce alterations of the subchondral bone in the mid- and long-term, yet very little is known about their onset. Possibly, these changes may be avoided by an enhanced microfracture technique with additional application of bone marrow aspirate. In this study, full-thickness chondral defects in the knee joints of minipigs were either treated with (1) debridement down to the subchondral bone plate alone, (2) debridement with microfracture, or (3) microfracture with additional application of bone marrow aspirate. At 4 weeks after microfracture, the loss of subchondral bone below the defects largely exceeded the original microfracture holes. Of note, a significant increase of osteoclast density was identified in defects treated with microfracture alone compared with debridement only. Both changes were significantly counteracted by the adjunct treatment with bone marrow. Debridement and microfracture without or with bone marrow were equivalent regarding the early cartilage repair. These data suggest that microfracture induced a substantial early resorption of the subchondral bone and also highlight the potential value of bone marrow aspirate as an adjunct to counteract these alterations. Clinical studies are warranted to further elucidate early events of osteochondral repair and the effect of enhanced microfracture techniques.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Small-Diameter Awls Improve Articular Cartilage Repair After Microfracture Treatment in a Translational Animal Model.Am J Sports Med. 2016 Jan;44(1):209-19. doi: 10.1177/0363546515610507. Epub 2015 Nov 6. Am J Sports Med. 2016. PMID: 26546301
-
Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair.J Orthop Res. 2009 Nov;27(11):1432-8. doi: 10.1002/jor.20905. J Orthop Res. 2009. PMID: 19402150
-
Subchondral Drilling Independent of Drill Hole Number Improves Articular Cartilage Repair and Reduces Subchondral Bone Alterations Compared With Debridement in Adult Sheep.Am J Sports Med. 2022 Aug;50(10):2669-2679. doi: 10.1177/03635465221104775. Epub 2022 Jul 14. Am J Sports Med. 2022. PMID: 35834876
-
Autologous tissue transplantations for osteochondral repair.Dan Med J. 2016 Apr;63(4):B5236. Dan Med J. 2016. PMID: 27034191 Review.
-
Microfracture: indications, technique, and results.Instr Course Lect. 2007;56:419-28. Instr Course Lect. 2007. PMID: 17472325 Review.
Cited by
-
Second-look arthroscopic and magnetic resonance analysis after internal fixation of osteochondral lesions of the talus.Sci Rep. 2022 Jun 27;12(1):10833. doi: 10.1038/s41598-022-14990-5. Sci Rep. 2022. PMID: 35760944 Free PMC article.
-
A Photopolymerizable Biocompatible Hyaluronic Acid Hydrogel Promotes Early Articular Cartilage Repair in a Minipig Model In Vivo.Adv Healthc Mater. 2023 Oct;12(26):e2300931. doi: 10.1002/adhm.202300931. Epub 2023 Aug 18. Adv Healthc Mater. 2023. PMID: 37567219 Free PMC article.
-
Sheep condyle model evaluation of bone marrow cell concentrate combined with a scaffold for repair of large osteochondral defects.Bone Joint Res. 2021 Oct;10(10):677-689. doi: 10.1302/2046-3758.1010.BJR-2020-0504.R1. Bone Joint Res. 2021. PMID: 34665001 Free PMC article.
-
Effects of different microfracture drilling parameters on bone quality: a finite element analysis.Front Bioeng Biotechnol. 2025 Jan 8;12:1515136. doi: 10.3389/fbioe.2024.1515136. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39845370 Free PMC article.
-
Cyst formation in the subchondral bone following cartilage repair.Clin Transl Med. 2020 Dec;10(8):e248. doi: 10.1002/ctm2.248. Clin Transl Med. 2020. PMID: 33377663 Free PMC article. Review.
References
-
- Gomoll A. H., Farr J., Gillogly S. D., Kercher J. & Minas T. Surgical Management of Articular Cartilage Defects of the Knee. J. Bone Joint Surg. Am. 92A, 2470–2490 (2010). - PubMed
-
- Steadman J. R., Rodkey W. G. & Rodrigo J. J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res., S362–369 (2001). - PubMed
-
- Erggelet C. Enhanced Marrow Stimulation Techniques for Cartilage Repair. Oper. Tech. Orthop. 24, 2–13 (2014).
-
- Fortier L. A. et al.. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J. Bone Joint Surg. Am. 92, 1927–1937 (2010). - PubMed
-
- Hoemann C. D. et al.. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J. Bone Joint Surg. Am. 87A, 2671–2686 (2005). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

