Liposomes loaded with bioactive lipids enhance antibacterial innate immunity irrespective of drug resistance
- PMID: 28345623
- PMCID: PMC5366871
- DOI: 10.1038/srep45120
Liposomes loaded with bioactive lipids enhance antibacterial innate immunity irrespective of drug resistance
Abstract
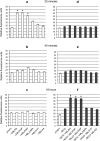
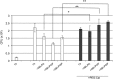
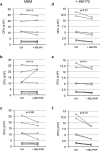
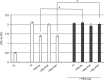
Phagocytosis is a key mechanism of innate immunity, and promotion of phagosome maturation may represent a therapeutic target to enhance antibacterial host response. Phagosome maturation is favored by the timely and coordinated intervention of lipids and may be altered in infections. Here we used apoptotic body-like liposomes (ABL) to selectively deliver bioactive lipids to innate cells, and then tested their function in models of pathogen-inhibited and host-impaired phagosome maturation. Stimulation of macrophages with ABLs carrying phosphatidic acid (PA), phosphatidylinositol 3-phosphate (PI3P) or PI5P increased intracellular killing of BCG, by inducing phagosome acidification and ROS generation. Moreover, ABLs carrying PA or PI5P enhanced ROS-mediated intracellular killing of Pseudomonas aeruginosa, in macrophages expressing a pharmacologically-inhibited or a naturally-mutated cystic fibrosis transmembrane conductance regulator. Finally, we show that bronchoalveolar lavage cells from patients with drug-resistant pulmonary infections increased significantly their capacity to kill in vivo acquired bacterial pathogens when ex vivo stimulated with PA- or PI5P-loaded ABLs. Altogether, these results provide the proof of concept of the efficacy of bioactive lipids delivered by ABL to enhance phagosome maturation dependent antimicrobial response, as an additional host-directed strategy aimed at the control of chronic, recurrent or drug-resistant infections.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Yeung T. & Grinstein S. Lipid signaling and the modulation of surface charge during phagocytosis. Immunol. Rev. 219, 17–36 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

