Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy
- PMID: 28345650
- PMCID: PMC5378974
- DOI: 10.1038/ncomms14754
Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy
Abstract
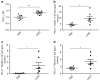
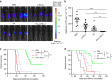
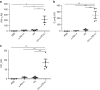
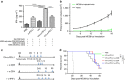
Both anti-PD1/PD-L1 therapy and oncolytic virotherapy have demonstrated promise, yet have exhibited efficacy in only a small fraction of cancer patients. Here we hypothesized that an oncolytic poxvirus would attract T cells into the tumour, and induce PD-L1 expression in cancer and immune cells, leading to more susceptible targets for anti-PD-L1 immunotherapy. Our results demonstrate in colon and ovarian cancer models that an oncolytic vaccinia virus attracts effector T cells and induces PD-L1 expression on both cancer and immune cells in the tumour. The dual therapy reduces PD-L1+ cells and facilitates non-redundant tumour infiltration of effector CD8+, CD4+ T cells, with increased IFN-γ, ICOS, granzyme B and perforin expression. Furthermore, the treatment reduces the virus-induced PD-L1+ DC, MDSC, TAM and Treg, as well as co-inhibitory molecules-double-positive, severely exhausted PD-1+CD8+ T cells, leading to reduced tumour burden and improved survival. This combinatorial therapy may be applicable to a much wider population of cancer patients.
Conflict of interest statement
D.L.B. is a shareholder of Sillajen Biotherapeutics, a company developing oncolytic viruses. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Combination of an oncolytic virus with PD-L1 blockade keeps cancer in check.Sci Transl Med. 2017 Apr 19;9(386):eaan2781. doi: 10.1126/scitranslmed.aan2781. Sci Transl Med. 2017. PMID: 28424330 Free PMC article.
References
-
- Lichty B. D., Breitbach C. J., Stojdl D. F. & Bell J. C. Going viral with cancer immunotherapy. Nat. Rev. Cancer 14, 559–567 (2014). - PubMed
-
- Andtbacka R. H. et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J. clin. oncol. 33, 2780–2788 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

