A modular and adaptive mass spectrometry-based platform for support of bioprocess development toward optimal host cell protein clearance
- PMID: 28346045
- PMCID: PMC5419088
- DOI: 10.1080/19420862.2017.1303023
A modular and adaptive mass spectrometry-based platform for support of bioprocess development toward optimal host cell protein clearance
Abstract
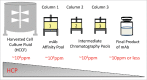
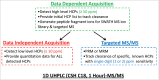
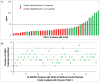
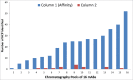
A modular and adaptive mass spectrometry (MS)-based platform was developed to provide fast, robust and sensitive host cell protein (HCP) analytics to support process development. This platform relies on one-dimensional ultra-high performance liquid chromatography (1D UHPLC) combined with several different MS data acquisition strategies to meet the needs of purification process development. The workflow was designed to allow HCP composition and quantitation for up to 20 samples per day, a throughput considered essential for real time bioprocess development support. With data-dependent acquisition (DDA), the 1D UHPLC-MS/MS method had excellent speed and demonstrated robustness in detecting unknown HCPs at ≥ 50 ng/mg (ppm) level. Combining 1D UHPLC with sequential window acquisition of all theoretical spectra (SWATH) MS enabled simultaneous detection and quantitation of all HCPs in single-digit ng/mg range within 1 hour, demonstrating for the first time the benefit of SWATH MS as a technique for HCP analysis. As another alternative, a targeted MS approach can be used to track the clearance of specific known HCP under various process conditions. This study highlights the importance of designing a robust LC-MS/MS workflow that not only allows HCP discovery, but also affords greatly improved process knowledge and capability in HCP removal. As an orthogonal and complementary detection approach to traditional HCP analysis by enzyme-linked immunosorbent assay, the reported LC-MS/MS workflow supports the development of bioprocesses with optimal HCP clearance and the production of safe and high quality therapeutic biopharmaceuticals.
Keywords: Bioprocess development; SWATH; host cell protein; mass spectrometry.
Figures
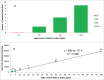
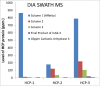
References
-
- ICH ICH harmonised tripartite guideline. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products Q6B. 1999.
-
- Shukla AA, Jiang C, Ma J, Rubacha M, Flansburg L, Lee SS. Demonstration of robust host cell protein clearance in biopharmaceutical downstream processes. Biotechnol Prog 2008; 24:615-22; PMID:18410156; http://dx.doi.org/ 10.1021/bp070396j - DOI - PubMed
-
- Tscheliessnig AL, Konrath J, Bates R, Jungbauer A. Host cell protein analysis in therapeutic protein bioprocessing - methods and applications. Biotechnol J 2013; 8:655-70; PMID:23436780; http://dx.doi.org/ 10.1002/biot.201200018 - DOI - PubMed
-
- Zhu-Shimoni J, Yu C, Nishihara J, Wong RM, Gunawan F, Lin M, Krawitz D, Liu P, Sandoval W, Vanderlaan M. Host cell protein testing by ELISAs and the use of orthogonal methods. Biotechnol Bioeng 2014; 111:2367-79; PMID:24995961; http://dx.doi.org/ 10.1002/bit.25327 - DOI - PubMed
-
- Vanderlaan M, Sandoval W, Liu P, Nishihara J, Tsui G, Lin M, Gunawan F, Parker S, Wong RM, Low J, et al.. Hamster phospholipase b-like 2 (PLBL2): A host cell proten impurity in therapeutic monoclonal antibodies derive from Chinese hamster ovary cells. Bioprocess Int 2015; 13:18-22.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
