The CSF Immune Response in HIV-1-Associated Cryptococcal Meningitis: Macrophage Activation, Correlates of Disease Severity, and Effect of Antiretroviral Therapy
- PMID: 28346317
- PMCID: PMC5469563
- DOI: 10.1097/QAI.0000000000001382
The CSF Immune Response in HIV-1-Associated Cryptococcal Meningitis: Macrophage Activation, Correlates of Disease Severity, and Effect of Antiretroviral Therapy
Abstract
Background: Immune modulation may improve outcome in HIV-associated cryptococcal meningitis. Animal studies suggest alternatively activated macrophages are detrimental but human studies are limited. We performed a detailed assessment of the cerebrospinal fluid (CSF) immune response and examined immune correlates of disease severity and poor outcome, and the effects of antiretroviral therapy (ART).
Methodology: We enrolled persons ≥18 years with first episode of HIV-associated cryptococcal meningitis. CSF immune response was assessed using flow cytometry and multiplex cytokine analysis. Principal component analysis was used to examine relationships between immune response, fungal burden, intracranial pressure and mortality, and the effects of recent ART initiation (<12 weeks).
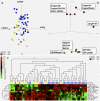
Findings: CSF was available from 57 persons (median CD4 34/μL). CD206 (alternatively activated macrophage marker) was expressed on 54% CD14 and 35% CD14 monocyte-macrophages. High fungal burden was not associated with CD206 expression but with a paucity of CD4, CD8, and CD4CD8 T cells and lower interleukin-6, G-CSF, and interleukin-5 concentrations. High intracranial pressure (≥30 cm H2O) was associated with fewer T cells, a higher fungal burden, and larger Cryptococcus organisms. Mortality was associated with reduced interferon-gamma concentrations and CD4CD8 T cells but lost statistical significance when adjusted for multiple comparisons. Recent ART was associated with increased CSF CD4/CD8 ratio and a significantly increased macrophage expression of CD206.
Conclusions: Paucity of CSF T cell infiltrate rather than alternative macrophage activation was associated with severe disease in HIV-associated cryptococcosis. ART had a pronounced effect on the immune response at the site of disease.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



References
-
- Emmons CW. Saprophytic sources of Cryptococcus neoformans associated with the pigeon (Columba livia). Am J Hyg. 1955;62:227–232. - PubMed
-
- Goldman DL, Khine H, Abadi J, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107:E66. - PubMed
-
- Salyer WR, Salyer DC, Baker RD. Primary complex of cryptococcus and pulmonary lymph nodes. J Infect Dis. 1974;130:74–77. - PubMed
-
- Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2004;72:99–118. - PubMed
-
- Cohen DB, Zijlstra EE, Mukaka M, et al. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop Med Int Health. 2010;15:910–917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

