New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation
- PMID: 28346364
- PMCID: PMC6154534
- DOI: 10.3390/molecules22040507
New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation
Abstract
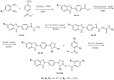
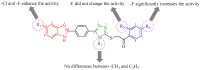
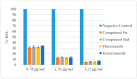
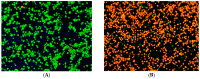
Owing to the growing need for antifungal agents, we synthesized a new series 2-((5-(4-(5-substituted-1H-benzimidazol-2-yl)phenyl)-4-substituted-4H-1,2,4-triazol-3-yl)thio)-1-(substitutedphenyl)ethan-1-one derivatives, which were tested against Candida species. The synthesized compounds were characterized and elucidated by FT-IR, ¹H-NMR, 13C-NMR and HR-MS spectroscopies. The synthesized compounds were screened in vitro anticandidal activity against Candida species by broth microdiluation methods. In vitro cytotoxic effects of the final compounds were determined by MTT assay. Microbiological studies revealed that compounds 5m, 5o, 5r, 5t, 5y, 5ab, and 5ad possess a good antifungal profile. Compounds 5w was the most active derivative and showed comparable antifungal activity to those of reference drugs ketoconazole and fluconazole. Cytotoxicity evaluation of compounds 5m, 5o, 5r, 5w, 5y, 5ab and 5ad showed that compounds 5w and 5ad were the least cytotoxic agents. Effects of these two compounds against ergosterol biosynthesis were observed by LC-MS-MS method, which is based on quantification of ergosterol level in C. albicans. Compounds 5w and 5d inhibited ergosterol biosynthesis concentration dependently. A fluorescence microscopy study was performed to visualize effect of compound 5w against C. albicans at cellular level. It was determined that compound 5w has a membrane damaging effect, which may be related with inhibition of biosynthesis of ergosterol.
Keywords: 1,2,4-triazole; NIH/3T3; antifungal activity; cytotoxicity; ergosterol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synthesis and Anticandidal Activity of New Imidazole-Chalcones.Molecules. 2018 Apr 4;23(4):831. doi: 10.3390/molecules23040831. Molecules. 2018. PMID: 29617329 Free PMC article.
-
Synthesis and Anticandidal Activity Evaluation of New Benzimidazole-Thiazole Derivatives.Molecules. 2017 Nov 23;22(12):2051. doi: 10.3390/molecules22122051. Molecules. 2017. PMID: 29168743 Free PMC article.
-
Synthesis and Antifungal Potential of Some Novel Benzimidazole-1,3,4-Oxadiazole Compounds.Molecules. 2019 Jan 6;24(1):191. doi: 10.3390/molecules24010191. Molecules. 2019. PMID: 30621357 Free PMC article.
-
Synthesis of Oxadiazole-Thiadiazole Hybrids and Their Anticandidal Activity.Molecules. 2017 Nov 18;22(11):2004. doi: 10.3390/molecules22112004. Molecules. 2017. PMID: 29156575 Free PMC article.
-
An Antifungal Benzimidazole Derivative Inhibits Ergosterol Biosynthesis and Reveals Novel Sterols.Antimicrob Agents Chemother. 2015 Oct;59(10):6296-307. doi: 10.1128/AAC.00640-15. Epub 2015 Jul 27. Antimicrob Agents Chemother. 2015. PMID: 26248360 Free PMC article.
Cited by
-
Microbial Transformation of Hesperidin and Biological Evaluation.ACS Omega. 2023 Nov 1;8(45):42610-42621. doi: 10.1021/acsomega.3c05334. eCollection 2023 Nov 14. ACS Omega. 2023. PMID: 38024700 Free PMC article.
-
1,2,4-Triazoles as Important Antibacterial Agents.Pharmaceuticals (Basel). 2021 Mar 7;14(3):224. doi: 10.3390/ph14030224. Pharmaceuticals (Basel). 2021. PMID: 33799936 Free PMC article. Review.
-
Design, Synthesis and Biological Evaluation of Novel N-Pyridyl-Hydrazone Derivatives as Potential Monoamine Oxidase (MAO) Inhibitors.Molecules. 2018 Jan 8;23(1):113. doi: 10.3390/molecules23010113. Molecules. 2018. PMID: 29316677 Free PMC article.
-
Benzimidazole chemistry in oncology: recent developments in synthesis, activity, and SAR analysis.RSC Adv. 2025 Jun 3;15(23):18593-18647. doi: 10.1039/d5ra01077b. eCollection 2025 May 29. RSC Adv. 2025. PMID: 40463335 Free PMC article. Review.
-
Synthesis and Anticandidal Activity of New Imidazole-Chalcones.Molecules. 2018 Apr 4;23(4):831. doi: 10.3390/molecules23040831. Molecules. 2018. PMID: 29617329 Free PMC article.
References
-
- Jiang Y., Cao Y., Zhang J., Zou Y., Chai X., Hu H., Zhao Q., Wu Q., Zhang D., Jiang Y., et al. Design, synthesis and antifungal evaluation of 1-(2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propyl)-1H-1,2,4-triazol-5(4H)-one. Eur. J. Med. Chem. 2011;46:3135–3141. doi: 10.1016/j.ejmech.2011.02.001. - DOI - PubMed
-
- Altıntop M.D., Kaplancıklı Z.A., Turan-Zitouni G., Özdemir A., İşcan G., Akalın G., Yıldırım Ş. Synthesis and anticandidal activity of new triazolothiadiazine derivatives. Eur. J. Med. Chem. 2011;46:5562–5566. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

