T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy
- PMID: 28346400
- PMCID: PMC5446300
- DOI: 10.1038/labinvest.2017.25
T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy
Abstract
Advances in cancer immunotherapy have offered new hope for patients with metastatic disease. This unfolding success story has been exemplified by a growing arsenal of novel immunotherapeutics, including blocking antibodies targeting immune checkpoint pathways, cancer vaccines, and adoptive cell therapy (ACT). Nonetheless, clinical benefit remains highly variable and patient-specific, in part, because all immunotherapeutic regimens vitally hinge on the capacity of endogenous and/or adoptively transferred T-effector (Teff) cells, including chimeric antigen receptor (CAR) T cells, to home efficiently into tumor target tissue. Thus, defects intrinsic to the multi-step T-cell homing cascade have become an obvious, though significantly underappreciated contributor to immunotherapy resistance. Conspicuous have been low intralesional frequencies of tumor-infiltrating T-lymphocytes (TILs) below clinically beneficial threshold levels, and peripheral rather than deep lesional TIL infiltration. Therefore, a Teff cell 'homing deficit' may arguably represent a dominant factor responsible for ineffective immunotherapeutic outcomes, as tumors resistant to immune-targeted killing thrive in such permissive, immune-vacuous microenvironments. Fortunately, emerging data is shedding light into the diverse mechanisms of immune escape by which tumors restrict Teff cell trafficking and lesional penetrance. In this review, we scrutinize evolving knowledge on the molecular determinants of Teff cell navigation into tumors. By integrating recently described, though sporadic information of pivotal adhesive and chemokine homing signatures within the tumor microenvironment with better established paradigms of T-cell trafficking under homeostatic or infectious disease scenarios, we seek to refine currently incomplete models of Teff cell entry into tumor tissue. We further summarize how cancers thwart homing to escape immune-mediated destruction and raise awareness of the potential impact of immune checkpoint blockers on Teff cell homing. Finally, we speculate on innovative therapeutic opportunities for augmenting Teff cell homing capabilities to improve immunotherapy-based tumor eradication in cancer patients, with special focus on malignant melanoma.
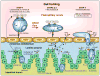
Figures



References
-
- Houot R, Schultz LM, Marabelle A, et al. T-cell-based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer Immunol Res. 2015;3:1115–1122. - PubMed
-
- Lee N, Zakka LR, Mihm MC, Jr, et al. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology. 2016;48:177–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

