Contribution of small conductance K+ channels to sinoatrial node pacemaker activity: insights from atrial-specific Na+ /Ca2+ exchange knockout mice
- PMID: 28346695
- PMCID: PMC5471364
- DOI: 10.1113/JP274249
Contribution of small conductance K+ channels to sinoatrial node pacemaker activity: insights from atrial-specific Na+ /Ca2+ exchange knockout mice
Abstract
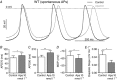
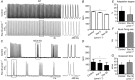
Key points: Repolarizing currents through K+ channels are essential for proper sinoatrial node (SAN) pacemaking, but the influence of intracellular Ca2+ on repolarization in the SAN is uncertain. We identified all three isoforms of Ca2+ -activated small conductance K+ (SK) channels in the murine SAN. SK channel blockade slows repolarization and subsequent depolarization of SAN cells. In the atrial-specific Na+ /Ca2+ exchanger (NCX) knockout mouse, cellular Ca2+ accumulation during spontaneous SAN pacemaker activity produces intermittent hyperactivation of SK channels, leading to arrhythmic pauses alternating with bursts of pacing. These findings suggest that Ca2+ -sensitive SK channels can translate changes in cellular Ca2+ into a repolarizing current capable of modulating pacemaking. SK channels are a potential pharmacological target for modulating SAN rate or treating SAN dysfunction, particularly under conditions characterized by abnormal increases in diastolic Ca2+ .
Abstract: Small conductance K+ (SK) channels have been implicated as modulators of spontaneous depolarization and electrical conduction that may be involved in cardiac arrhythmia. However, neither their presence nor their contribution to sinoatrial node (SAN) pacemaker activity has been investigated. Using quantitative PCR (q-PCR), immunostaining and patch clamp recordings of membrane current and voltage, we identified all three SK isoforms (SK1, SK2 and SK3) in mouse SAN. Inhibition of SK channels with the specific blocker apamin prolonged action potentials (APs) in isolated SAN cells. Apamin also slowed diastolic depolarization and reduced pacemaker rate in isolated SAN cells and intact tissue. We investigated whether the Ca2+ -sensitive nature of SK channels could explain arrhythmic SAN pacemaker activity in the atrial-specific Na+ /Ca2+ exchange (NCX) knockout (KO) mouse, a model of cellular Ca2+ overload. SAN cells isolated from the NCX KO exhibited higher SK current than wildtype (WT) and apamin prolonged their APs. SK blockade partially suppressed the arrhythmic burst pacing pattern of intact NCX KO SAN tissue. We conclude that SK channels have demonstrable effects on SAN pacemaking in the mouse. Their Ca2+ -dependent activation translates changes in cellular Ca2+ into a repolarizing current capable of modulating regular pacemaking. This Ca2+ dependence also promotes abnormal automaticity when these channels are hyperactivated by elevated Ca2+ . We propose SK channels as a potential target for modulating SAN rate, and for treating patients affected by SAN dysfunction, particularly in the setting of Ca2+ overload.
Keywords: Na+/Ca2+ exchange; sinoatrial node; small conductance K+ channels.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures











References
-
- Chen WT, Chen YC, Lu YY, Kao YH, Huang JH, Lin YK, Chen SA & Chen YJ (2013). Apamin modulates electrophysiological characteristics of the pulmonary vein and the sinoatrial node. Eur J Clin Invest 43, 957–963. - PubMed
-
- Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sørensen US, Hansen RS & Grunnet M (2011). Effects on atrial fibrillation in aged hypertensive rats by Ca2+‐activated K+ channel inhibition. Hypertension 57, 1129–1135. - PubMed
-
- Diness JG, Sørensen US, Nissen JD, Al‐Shahib B, Jespersen T, Grunnet M & Hansen RS (2010). Inhibition of small‐conductance Ca2+‐activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol 3, 380–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

