Synthesis of Ordered Mesoporous CuO/CeO₂ Composite Frameworks as Anode Catalysts for Water Oxidation
- PMID: 28347106
- PMCID: PMC5304801
- DOI: 10.3390/nano5041971
Synthesis of Ordered Mesoporous CuO/CeO₂ Composite Frameworks as Anode Catalysts for Water Oxidation
Abstract
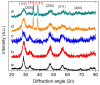
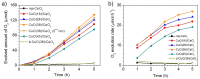
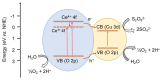
Cerium-rich metal oxide materials have recently emerged as promising candidates for the photocatalytic oxygen evolution reaction (OER). In this article, we report the synthesis of ordered mesoporous CuO/CeO₂ composite frameworks with different contents of copper(II) oxide and demonstrate their activity for photocatalytic O₂ production via UV-Vis light-driven oxidation of water. Mesoporous CuO/CeO₂ materials have been successfully prepared by a nanocasting route, using mesoporous silica as a rigid template. X-ray diffraction, electron transmission microscopy and N₂ porosimetry characterization of the as-prepared products reveal a mesoporous structure composed of parallel arranged nanorods, with a large surface area and a narrow pore size distribution. The molecular structure and optical properties of the composite materials were investigated with Raman and UV-Vis/NIR diffuse reflectance spectroscopy. Catalytic results indicated that incorporation of CuO clusters in the CeO₂ lattice improved the photochemical properties. As a result, the CuO/CeO₂ composite catalyst containing ~38 wt % CuO reaches a high O₂ evolution rate of ~19.6 µmol·h-1 (or 392 µmol·h-1·g-1) with an apparent quantum efficiency of 17.6% at λ = 365 ± 10 nm. This OER activity compares favorably with that obtained from the non-porous CuO/CeO₂ counterpart (~1.3 µmol·h-1) and pure mesoporous CeO₂ (~1 µmol·h-1).
Keywords: cerium oxide; mesoporous materials; nanocasting; nanostructured; water oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Combustion Synthesis of Non-Precious CuO-CeO₂ Nanocrystalline Catalysts with Enhanced Catalytic Activity for Methane Oxidation.Materials (Basel). 2019 Mar 15;12(6):878. doi: 10.3390/ma12060878. Materials (Basel). 2019. PMID: 30875991 Free PMC article.
-
Unique properties of ceria nanoparticles supported on metals: novel inverse ceria/copper catalysts for CO oxidation and the water-gas shift reaction.Acc Chem Res. 2013 Aug 20;46(8):1702-11. doi: 10.1021/ar300231p. Epub 2013 Jan 3. Acc Chem Res. 2013. PMID: 23286528
-
Remarkable Activity of Nanoarchitectonics Mesoporous CuO/CeO₂-TiO₂ Prepared by Nanocasting and Deposition Precipitation Techniques.J Nanosci Nanotechnol. 2020 May 1;20(5):2791-2802. doi: 10.1166/jnn.2020.17428. J Nanosci Nanotechnol. 2020. PMID: 31635615
-
Interactions of CO and/or H2O with mesoporous oxide-supported metal catalysts: the role of MSI effects.Chem Commun (Camb). 2025 Apr 15;61(32):5917-5929. doi: 10.1039/d5cc01012h. Chem Commun (Camb). 2025. PMID: 40145407 Review.
-
Macromolecular Architecture in the Synthesis of Micro- and Mesoporous Polymers.Polymers (Basel). 2024 Nov 24;16(23):3267. doi: 10.3390/polym16233267. Polymers (Basel). 2024. PMID: 39684011 Free PMC article. Review.
Cited by
-
Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure.Nanomaterials (Basel). 2020 Aug 31;10(9):1727. doi: 10.3390/nano10091727. Nanomaterials (Basel). 2020. PMID: 32878224 Free PMC article.
-
Frontiers in Mesoporous Nanomaterials.Nanomaterials (Basel). 2016 Jan 11;6(1):15. doi: 10.3390/nano6010015. Nanomaterials (Basel). 2016. PMID: 28344272 Free PMC article.
-
Interrelationships between the structural, spectroscopic, and antibacterial properties of nanoscale (< 50 nm) cerium oxides.Sci Rep. 2021 Oct 22;11(1):20875. doi: 10.1038/s41598-021-00222-9. Sci Rep. 2021. PMID: 34686704 Free PMC article.
-
Recent Advancements in the Fabrication of Functional Nanoporous Materials and Their Biomedical Applications.Materials (Basel). 2022 Mar 13;15(6):2111. doi: 10.3390/ma15062111. Materials (Basel). 2022. PMID: 35329563 Free PMC article. Review.
-
One-step electrodeposition of cerium-doped nickel hydroxide nanosheets for effective oxygen generation.RSC Adv. 2019 Jun 6;9(31):17891-17896. doi: 10.1039/c9ra02682g. eCollection 2019 Jun 4. RSC Adv. 2019. PMID: 35520549 Free PMC article.
References
-
- Subbaraman R., Tripkovic D., Chang K.C., Strmcnik D., Paulikas A.P., Hirunsit P., Chan M., Greeley J., Stamenkovic V., Markovic N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012;11:550–557. doi: 10.1038/nmat3313. - DOI - PubMed
-
- Nakamura R., Okamura T., Ohashi N., Imanishi A., Nakato Y. Molecular Mechanisms of Photoinduced Oxygen Evolution, PL Emission, and Surface Roughening at Atomically Smooth (110) and (100) n-TiO2 (Rutile) Surfaces in Aqueous Acidic Solutions. J. Am. Chem. Soc. 2005;127:12975–12983. doi: 10.1021/ja053252e. - DOI - PubMed
-
- Papadas I.T., Christodoulides J.A., Kioseoglou G., Armatas G.S. A high surface area ordered mesoporous BiFeO3 semiconductor with efficient water oxidation activity. J. Mater. Chem. A. 2015;3:1587–1593. doi: 10.1039/C4TA05272B. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

