Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics
- PMID: 28347114
- PMCID: PMC5304787
- DOI: 10.3390/nano5042169
Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics
Abstract
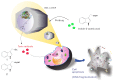
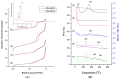
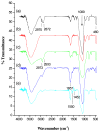
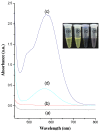
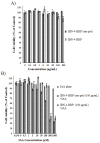
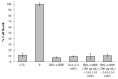
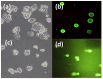
To develop a carrier for use in enzyme prodrug therapy, Horseradish peroxidase (HRP) was immobilized onto mesoporous silica nanoparticles (IBN-4: Institute of Bioengineering and Nanotechnology), where the nanoparticle surfaces were functionalized with 3-aminopropyltrimethoxysilane and further conjugated with glutaraldehyde. Consequently, the enzymes could be stabilized in nanochannels through the formation of covalent imine bonds. This strategy was used to protect HRP from immune exclusion, degradation and denaturation under biological conditions. Furthermore, immobilization of HRP in the nanochannels of IBN-4 nanomaterials exhibited good functional stability upon repetitive use and long-term storage (60 days) at 4 °C. The generation of functionalized and HRP-immobilized nanomaterials was further verified using various characterization techniques. The possibility of using HRP-encapsulated IBN-4 materials in prodrug cancer therapy was also demonstrated by evaluating their ability to convert a prodrug (indole-3- acetic acid (IAA)) into cytotoxic radicals, which triggered tumor cell apoptosis in human colon carcinoma (HT-29 cell line) cells. A lactate dehydrogenase (LDH) assay revealed that cells could be exposed to the IBN-4 nanocomposites without damaging their membranes, confirming apoptotic cell death. In summary, we demonstrated the potential of utilizing large porous mesoporous silica nanomaterials (IBN-4) as enzyme carriers for prodrug therapy.
Keywords: Institute of Bioengineering and Nanotechnology (IBN-4); anti-cancer; enzyme immobilization; mesoporous silica nanoparticles; prodrug therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Conquering Cancer Multi-Drug Resistance Using Curcumin and Cisplatin Prodrug-Encapsulated Mesoporous Silica Nanoparticles for Synergistic Chemo- and Photodynamic Therapies.Nanomaterials (Basel). 2022 Oct 21;12(20):3693. doi: 10.3390/nano12203693. Nanomaterials (Basel). 2022. PMID: 36296885 Free PMC article.
-
Effective immobilization of lactate dehydrogenase onto mesoporous silica.Biotechnol Appl Biochem. 2022 Dec;69(6):2550-2560. doi: 10.1002/bab.2304. Epub 2022 Feb 24. Biotechnol Appl Biochem. 2022. PMID: 34962677
-
Co-immobilization of enzymes with the help of a dendronized polymer and mesoporous silica nanoparticles.J Mater Chem B. 2015 Aug 14;3(30):6174-6184. doi: 10.1039/c5tb00543d. Epub 2015 Jul 7. J Mater Chem B. 2015. PMID: 32262736
-
Enzyme immobilization onto the nanomaterials: Application in enzyme stability and prodrug-activated cancer therapy.Int J Biol Macromol. 2020 Jan 15;143:665-676. doi: 10.1016/j.ijbiomac.2019.12.064. Epub 2019 Dec 10. Int J Biol Macromol. 2020. PMID: 31830450 Review.
-
Mesoporous Silica Nanomaterials: Versatile Nanocarriers for Cancer Theranostics and Drug and Gene Delivery.Pharmaceutics. 2019 Feb 13;11(2):77. doi: 10.3390/pharmaceutics11020077. Pharmaceutics. 2019. PMID: 30781850 Free PMC article. Review.
Cited by
-
Enzyme-polymeric/inorganic metal oxide/hybrid nanoparticle bio-conjugates in the development of therapeutic and biosensing platforms.J Adv Res. 2021 Feb 4;33:227-239. doi: 10.1016/j.jare.2021.01.012. eCollection 2021 Nov. J Adv Res. 2021. PMID: 34603792 Free PMC article. Review.
-
Adenosine triphosphate-activated prodrug system for on-demand bacterial inactivation and wound disinfection.Nat Commun. 2022 Aug 11;13(1):4712. doi: 10.1038/s41467-022-32453-3. Nat Commun. 2022. PMID: 35953495 Free PMC article.
-
Horseradish Peroxidase-Functionalized Gold Nanoconjugates for Breast Cancer Treatment Based on Enzyme Prodrug Therapy.Int J Nanomedicine. 2022 Jan 26;17:409-422. doi: 10.2147/IJN.S323802. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35115775 Free PMC article.
-
The autophagic inhibition oral squamous cell carcinoma cancer growth of 16-hydroxy-cleroda-3,14-dine-15,16-olide.Oncotarget. 2017 Jul 4;8(45):78379-78396. doi: 10.18632/oncotarget.18987. eCollection 2017 Oct 3. Oncotarget. 2017. PMID: 29108236 Free PMC article.
-
Frontiers in Mesoporous Nanomaterials.Nanomaterials (Basel). 2016 Jan 11;6(1):15. doi: 10.3390/nano6010015. Nanomaterials (Basel). 2016. PMID: 28344272 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

