Electric Field-Responsive Mesoporous Suspensions: A Review
- PMID: 28347119
- PMCID: PMC5304764
- DOI: 10.3390/nano5042249
Electric Field-Responsive Mesoporous Suspensions: A Review
Abstract
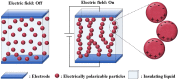
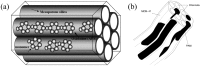
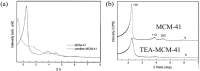
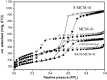
This paper briefly reviews the fabrication and electrorheological (ER) characteristics of mesoporous materials and their nanocomposites with conducting polymers under an applied electric field when dispersed in an insulating liquid. Smart fluids of electrically-polarizable particles exhibit a reversible and tunable phase transition from a liquid-like to solid-like state in response to an external electric field of various strengths, and have potential applications in a variety of active control systems. The ER properties of these mesoporous suspensions are explained further according to their dielectric spectra in terms of the flow curve, dynamic moduli, and yield stress.
Keywords: electrorheology; mesoporous; nanocomposite; suspension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



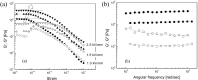
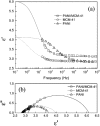
References
-
- Wu X., Huang W.M., Zhao Y., Ding Z., Tang C., Zhang J. Mechanisms of the shape memory effect in polymeric materials. Polymers. 2013;5:1169–1202. doi: 10.3390/polym5041169. - DOI
-
- Klingenberg D.J., Zukoski C.F. Studies on the steady-shear behavior of electrorheological suspensions. Langmuir. 1990;6:15–24. doi: 10.1021/la00091a003. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

