Genome-wide identification of conserved intronic non-coding sequences using a Bayesian segmentation approach
- PMID: 28347272
- PMCID: PMC5369223
- DOI: 10.1186/s12864-017-3645-2
Genome-wide identification of conserved intronic non-coding sequences using a Bayesian segmentation approach
Abstract
Background: Computational identification of non-coding RNAs (ncRNAs) is a challenging problem. We describe a genome-wide analysis using Bayesian segmentation to identify intronic elements highly conserved between three evolutionarily distant vertebrate species: human, mouse and zebrafish. We investigate the extent to which these elements include ncRNAs (or conserved domains of ncRNAs) and regulatory sequences.
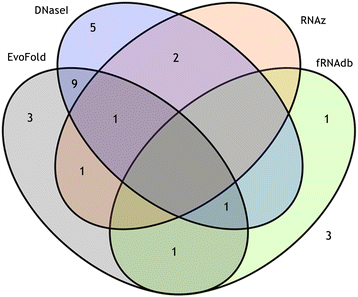
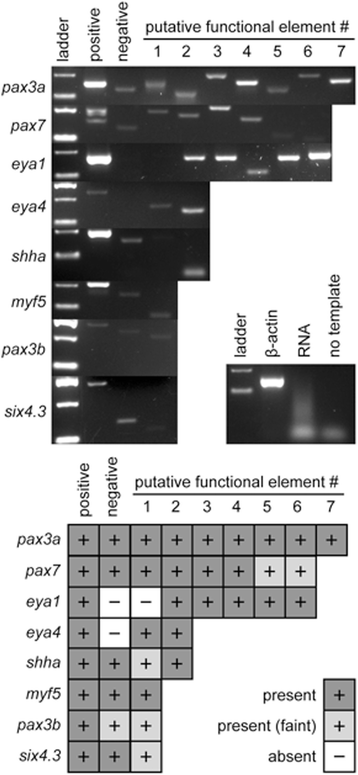
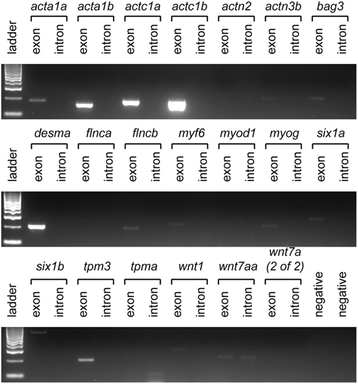
Results: We identified 655 deeply conserved intronic sequences in a genome-wide analysis. We also performed a pathway-focussed analysis on genes involved in muscle development, detecting 27 intronic elements, of which 22 were not detected in the genome-wide analysis. At least 87% of the genome-wide and 70% of the pathway-focussed elements have existing annotations indicative of conserved RNA secondary structure. The expression of 26 of the pathway-focused elements was examined using RT-PCR, providing confirmation that they include expressed ncRNAs. Consistent with previous studies, these elements are significantly over-represented in the introns of transcription factors.
Conclusions: This study demonstrates a novel, highly effective, Bayesian approach to identifying conserved non-coding sequences. Our results complement previous findings that these sequences are enriched in transcription factors. However, in contrast to previous studies which suggest the majority of conserved sequences are regulatory factor binding sites, the majority of conserved sequences identified using our approach contain evidence of conserved RNA secondary structures, and our laboratory results suggest most are expressed. Functional roles at DNA and RNA levels are not mutually exclusive, and many of our elements possess evidence of both. Moreover, ncRNAs play roles in transcriptional and post-transcriptional regulation, and this may contribute to the over-representation of these elements in introns of transcription factors. We attribute the higher sensitivity of the pathway-focussed analysis compared to the genome-wide analysis to improved alignment quality, suggesting that enhanced genomic alignments may reveal many more conserved intronic sequences.
Keywords: Bayesian modelling; Conserved non-coding sequences; Genome segmentation; Putative functional elements; ncRNA.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

