Design of a randomized controlled trial of physical training and cancer (Phys-Can) - the impact of exercise intensity on cancer related fatigue, quality of life and disease outcome
- PMID: 28347291
- PMCID: PMC5368942
- DOI: 10.1186/s12885-017-3197-5
Design of a randomized controlled trial of physical training and cancer (Phys-Can) - the impact of exercise intensity on cancer related fatigue, quality of life and disease outcome
Abstract
Background: Cancer-related fatigue is a common problem in persons with cancer, influencing health-related quality of life and causing a considerable challenge to society. Current evidence supports the beneficial effects of physical exercise in reducing fatigue, but the results across studies are not consistent, especially in terms of exercise intensity. It is also unclear whether use of behaviour change techniques can further increase exercise adherence and maintain physical activity behaviour. This study will investigate whether exercise intensity affects fatigue and health related quality of life in persons undergoing adjuvant cancer treatment. In addition, to examine effects of exercise intensity on mood disturbance, adherence to oncological treatment, adverse effects from treatment, activities of daily living after treatment completion and return to work, and behaviour change techniques effect on exercise adherence. We will also investigate whether exercise intensity influences inflammatory markers and cytokines, and whether gene expressions following training serve as mediators for the effects of exercise on fatigue and health related quality of life.
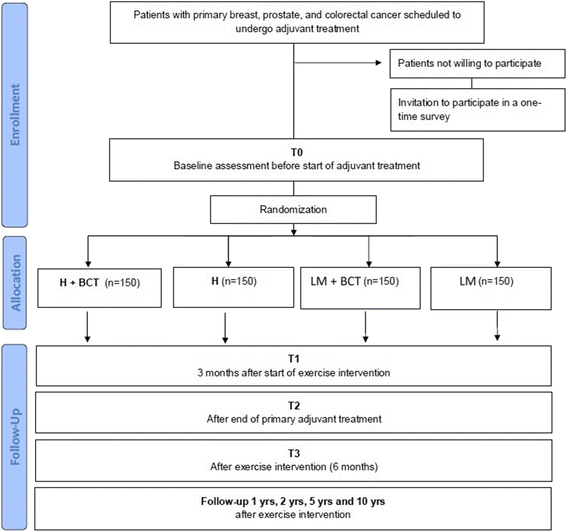
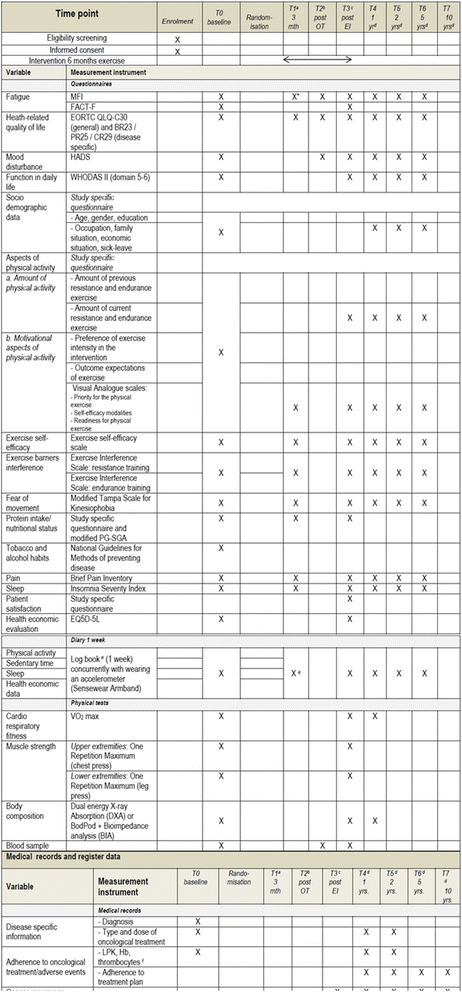
Methods/design: Six hundred newly diagnosed persons with breast, colorectal or prostate cancer undergoing adjuvant therapy will be randomized in a 2 × 2 factorial design to following conditions; A) individually tailored low-to-moderate intensity exercise with or without behaviour change techniques or B) individually tailored high intensity exercise with or without behaviour change techniques. The training consists of both resistance and endurance exercise sessions under the guidance of trained coaches. The primary outcomes, fatigue and health related quality of life, are measured by self-reports. Secondary outcomes include fitness, mood disturbance, adherence to the cancer treatment, adverse effects, return to activities of daily living after completed treatment, return to work as well as inflammatory markers, cytokines and gene expression.
Discussion: The study will contribute to our understanding of the value of exercise and exercise intensity in reducing fatigue and improving health related quality of life and, potentially, clinical outcomes. The value of behaviour change techniques in terms of adherence to and maintenance of physical exercise behaviour in persons with cancer will be evaluated.
Trial registration: NCT02473003 , October, 2014.
Keywords: Behaviour change techniques; Biological mechanism; Cancer; Fatigue; Physical exercise; Quality of life; Randomized controlled trial.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

