Impact of school peanut-free policies on epinephrine administration
- PMID: 28347736
- PMCID: PMC5546995
- DOI: 10.1016/j.jaci.2017.01.040
Impact of school peanut-free policies on epinephrine administration
Abstract
Background: Children with food allergies spend a large proportion of time in school but characteristics of allergic reactions in schools are not well studied. Some schools self-designate as peanut-free or have peanut-free areas, but the impact of policies on clinical outcomes has not been evaluated.
Objective: We sought to determine the effect of peanut-free policies on rates of epinephrine administration for allergic reactions in Massachusetts public schools.
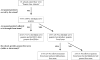
Methods: In this retrospective study, we analyzed (1) rates of epinephrine administration in all Massachusetts public schools and (2) Massachusetts public school nurse survey reports of school peanut-free policies from 2006 to 2011 and whether schools self-designated as "peanut-free" based on policies. Rates of epinephrine administration were compared for schools with or without peanut-restrictive policies.
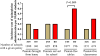
Results: The percentage of schools with peanut-restrictive policies did not change significantly in the study time frame. There was variability in policies used by schools self-designated as peanut-free. No policy was associated with complete absence of allergic reactions. Both self-designated peanut-free schools and schools banning peanuts from being served in school or brought from home reported allergic reactions to nuts. Policies restricting peanuts from home, served in schools, or having peanut-free classrooms did not affect epinephrine administration rates. Schools with peanut-free tables, compared to without, had lower rates of epinephrine administration (incidence rate per 10,000 students 0.2 and 0.6, respectively, P = .009).
Conclusions: These data provide a basis for evidence-based school policies for children with food allergies. Further studies are required before decisions can be made regarding peanut-free policies in schools.
Keywords: Peanut allergy; anaphylaxis; epinephrine; food allergy; school.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Peanut-free schools: What does it really mean, and are they necessary?J Allergy Clin Immunol. 2017 Aug;140(2):391-392. doi: 10.1016/j.jaci.2017.03.037. Epub 2017 Apr 25. J Allergy Clin Immunol. 2017. PMID: 28454741 No abstract available.
-
Epinephrine use as a measure of successful food allergy management.J Allergy Clin Immunol. 2017 Oct;140(4):1213-1214. doi: 10.1016/j.jaci.2017.06.035. Epub 2017 Aug 23. J Allergy Clin Immunol. 2017. PMID: 28844496 No abstract available.
-
Reply.J Allergy Clin Immunol. 2017 Oct;140(4):1214-1215. doi: 10.1016/j.jaci.2017.06.034. Epub 2017 Aug 23. J Allergy Clin Immunol. 2017. PMID: 28844497 No abstract available.
References
-
- Sicherer SH, Mahr T. American Academy of Pediatrics Section on A, Immunology. Management of food allergy in the school setting. Pediatrics. 2010;126(6):1232–9. - PubMed
-
- Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S116–25. - PubMed
-
- Longo G, Berti I, Burks AW, Krauss B, Barbi E. IgE-mediated food allergy in children. Lancet. 2013;382(9905):1656–64. - PubMed
-
- Shah SS, Parker CL, O’Brian Smith E, Davis CM. Disparity in the Availability of Injectable Epinephrine in a Large, Diverse US School District. J Allergy Clin Immunol Pract. 2014;2(3):288–93 e1. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

