Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases
- PMID: 28347855
- PMCID: PMC5610925
- DOI: 10.1016/j.jtos.2017.03.003
Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases
Abstract
Purpose: Coexistence of an ocular surface disease can mask the typical features of ocular surface squamous neoplasia (OSSN). The purpose of this study was to evaluate high resolution optical coherence tomography (HR-OCT) as an adjunct in the detection and differentiation of OSSN within coexisting ocular surface pathologies.
Methods: Retrospective study of 16 patients with ocular surface disease and lesions suspicious for OSSN that were evaluated with HR-OCT. HR-OCT images of the lesions were taken to look for evidence of OSSN. Biopsies were performed in all cases, and the HR-OCT findings were compared to the histological results.
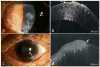
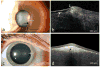
Results: Of the 16 patients with OSSN and a coexisting ocular surface disease, 12 were found to have OSSN by HR-OCT and all were subsequently confirmed by biopsy. Two patients had OSSN with rosacea, one with pingueculum, two within pterygia, one with Salzmann' nodular degeneration, six with limbal stem cell deficiency (LSCD)/scarring. In all 12 cases HR-OCT images revealed classical findings of hyper-reflective, thickened epithelium and an abrupt transition from normal to abnormal epithelium. OSSN was ruled out by HR-OCT in four cases (2 Salzmann's, 1 mucous membrane pemphigoid, and 1 LSCD). Negative findings were confirmed by biopsy. HR-OCT was used to follow resolution of the OSSN in positive cases, and it detected recurrence in 1 case.
Conclusions: While histopathology is the gold standard in the diagnosis of OSSN, HR-OCT can be used to noninvasively detect the presence of OSSN in patients with coexisting ocular conditions.
Keywords: Conjunctiva; Cornea; Lymphoma; Melanoma; Ocular surface squamous neoplasia (OSSN); Optical coherence tomography (OCT); Pterygium; Tumor.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no proprietary or commercial interest in any materials or concepts discussed in this article.
Figures





Similar articles
-
Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia.Ophthalmology. 2012 Mar;119(3):481-6. doi: 10.1016/j.ophtha.2011.08.028. Epub 2011 Dec 9. Ophthalmology. 2012. PMID: 22154538
-
High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology.Ocul Surf. 2015 Jul;13(3):226-35. doi: 10.1016/j.jtos.2015.02.001. Epub 2015 Apr 6. Ocul Surf. 2015. PMID: 26045235 Free PMC article. Review.
-
Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia.Ocul Surf. 2014 Jan;12(1):46-58. doi: 10.1016/j.jtos.2013.11.001. Epub 2013 Nov 9. Ocul Surf. 2014. PMID: 24439046 Free PMC article. Review.
-
Chronic inflammation as a proposed risk factor for ocular surface squamous neoplasia.Ocul Surf. 2024 Jul;33:1-10. doi: 10.1016/j.jtos.2024.02.006. Epub 2024 Mar 1. Ocul Surf. 2024. PMID: 38432640
-
Anterior Segment Swept-Source Optical Coherence Tomography in Ocular Surface Tumors and Simulating Lesions and Correlation With Histopathologic Diagnosis.Cornea. 2025 Mar 28;44(7):806-818. doi: 10.1097/ICO.0000000000003856. Cornea. 2025. PMID: 40152549
Cited by
-
Epidemiology and survival outcomes of patients with orbital region non-cutaneous squamous cell carcinoma: a population-based analysis.Front Oncol. 2023 May 5;13:1152337. doi: 10.3389/fonc.2023.1152337. eCollection 2023. Front Oncol. 2023. PMID: 37213302 Free PMC article.
-
Ocular surface squamous neoplasia: management and outcomes.Eye (Lond). 2021 Jun;35(6):1562-1573. doi: 10.1038/s41433-021-01422-3. Epub 2021 Feb 9. Eye (Lond). 2021. PMID: 33564137 Free PMC article. Review.
-
Imaging Techniques in the Diagnosis and Management of Ocular Tumors: Prospects and Challenges.AAPS J. 2018 Sep 5;20(6):97. doi: 10.1208/s12248-018-0259-9. AAPS J. 2018. PMID: 30187172 Review.
-
Salzmann nodular degeneration: prevalence, impact, and management strategies.Clin Ophthalmol. 2019 Jul 25;13:1305-1314. doi: 10.2147/OPTH.S166280. eCollection 2019. Clin Ophthalmol. 2019. PMID: 31413538 Free PMC article.
-
The use of high resolution optical coherence tomography (HR-OCT) in the diagnosis of ocular surface masqueraders.Ocul Surf. 2022 Apr;24:74-82. doi: 10.1016/j.jtos.2022.02.003. Epub 2022 Feb 26. Ocul Surf. 2022. PMID: 35231640 Free PMC article. Review.
References
-
- Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–50. - PubMed
-
- Grossniklaus HE, Green WR, Luckenbach M, Chan CC. Conjunctival lesions in adults. A clinical and histopathologic review. Cornea. 1987;6:78–116. - PubMed
-
- Basti S, Macsai MS. Ocular surface squamous neoplasia. Cornea. 2003;22:687–704. - PubMed
-
- Blodi FC. Squamous cell carcinoma of the conjunctiva. Doc Ophthalmol. 1973;34:93–108. - PubMed
-
- Kao AA, Galor A, Karp CL, Abdelaziz A, Feuer WJ, Dubovy SR. Clinicopathologic correlation of ocular surface squamous neoplasms at Bascom Palmer Eye Institute: 2001 to 2010. Ophthalmology. 2012;119:1773–6. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

