Porcine reproductive and respiratory syndrome virus (PRRSV) up-regulates IL-8 expression through TAK-1/JNK/AP-1 pathways
- PMID: 28347884
- PMCID: PMC7111726
- DOI: 10.1016/j.virol.2017.03.009
Porcine reproductive and respiratory syndrome virus (PRRSV) up-regulates IL-8 expression through TAK-1/JNK/AP-1 pathways
Abstract
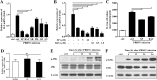
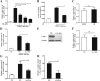
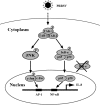
The acute phase of respiratory distress caused by porcine reproductive and respiratory syndrome virus (PRRSV) is likely a consequence of the release of inflammatory cytokines in the lung. IL-8, the main chemokine and activator of neutrophils, might be related to the lung injury upon PRRSV infection. In this study, we showed that PRRSV induced IL-8 expression in vivo and in vitro. Subsequently, we demonstrated that JNK and NF-κB pathways were activated upon PRRSV infection and required for the enhancement of IL-8 expression. We further verified that PRRSV-activated TAK-1 was essential for the activation of JNK and NF-κB pathways and IL-8 expression. Moreover, we revealed an AP-1 binding motif in the cloned porcine IL-8 (pIL-8) promoter, and deletion of this motif abolished the pIL-8 promoter activity. Finally, we found that the JNK-activated AP-1 subunit c-Jun was critical for the up-regulation of IL-8 expression by PRRSV. These data suggest that PRRSV-induced IL-8 production is likely through the TAK-1/JNK/AP-1 pathways.
Keywords: AP-1; IL-8; JNK; PRRSV; TAK-1.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) induces IL-6 production through TAK-1/JNK/AP-1 and TAK-1/NF-κB signaling pathways.Vet Microbiol. 2021 May;256:109061. doi: 10.1016/j.vetmic.2021.109061. Epub 2021 Mar 26. Vet Microbiol. 2021. PMID: 33836390
-
Porcine reproductive and respiratory syndrome virus (PRRSV) induces IL-12p40 production through JNK-AP-1 and NF-κB signaling pathways.Virus Res. 2016 Oct 2;225:73-81. doi: 10.1016/j.virusres.2016.09.009. Epub 2016 Sep 20. Virus Res. 2016. PMID: 27663131
-
Porcine reproductive and respiratory syndrome virus (PRRSV) up-regulates IL-15 through PKCβ1-TAK1-NF-κB signaling pathway.Virology. 2016 Sep;496:166-174. doi: 10.1016/j.virol.2016.06.007. Epub 2016 Jun 16. Virology. 2016. PMID: 27318153
-
Porcine reproductive and respiratory syndrome virus induces interleukin-1β through MyD88/ERK/AP-1 and NLRP3 inflammasome in microglia.Vet Microbiol. 2018 Dec;227:82-89. doi: 10.1016/j.vetmic.2018.10.030. Epub 2018 Oct 30. Vet Microbiol. 2018. PMID: 30473357
-
Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Induces Interleukin-17 Production via Activation of the IRAK1-PI3K-p38MAPK-C/EBPβ/CREB Pathways.J Virol. 2019 Oct 15;93(21):e01100-19. doi: 10.1128/JVI.01100-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413135 Free PMC article.
Cited by
-
Cellular Innate Immunity against PRRSV and Swine Influenza Viruses.Vet Sci. 2019 Mar 11;6(1):26. doi: 10.3390/vetsci6010026. Vet Sci. 2019. PMID: 30862035 Free PMC article. Review.
-
Proinflammatory Cytokine Changes in Bronchoalveolar Lavage Fluid Cells Isolated from Pigs Infected Solely with Porcine Reproductive and Respiratory Syndrome Virus or Co-infected with Swine Influenza Virus.J Vet Res. 2019 Oct 24;63(4):489-495. doi: 10.2478/jvetres-2019-0063. eCollection 2019 Dec. J Vet Res. 2019. PMID: 31934657 Free PMC article.
-
Signaling pathways involved in regulating apoptosis induction in host cells upon PRRSV infection.Virus Genes. 2019 Aug;55(4):433-439. doi: 10.1007/s11262-019-01665-z. Epub 2019 Apr 19. Virus Genes. 2019. PMID: 31004277 Review.
-
Porcine circovirus type 2 upregulates endothelial-derived IL-8 production in porcine iliac artery endothelial cells via the RIG-I/MDA-5/MAVS/JNK signaling pathway.BMC Vet Res. 2020 Jul 29;16(1):265. doi: 10.1186/s12917-020-02486-1. BMC Vet Res. 2020. PMID: 32727484 Free PMC article.
-
Molecular and Cellular Mechanisms for PRRSV Pathogenesis and Host Response to Infection.Virus Res. 2020 Sep;286:197980. doi: 10.1016/j.virusres.2020.197980. Epub 2020 Apr 18. Virus Res. 2020. PMID: 32311386 Free PMC article. Review.
References
-
- Anderson P., Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009;10(6):430–436. - PubMed
-
- Bottero V., Kerur N., Sadagopan S., Patel K., Sharma-Walia N., Chandran B. Phosphorylation and polyubiquitination of transforming growth factor beta-activated kinase 1 are necessary for activation of NF-kappaB by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J. Virol. 2011;85(5):1980–1993. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

