The role of metabolic states in development and disease
- PMID: 28347941
- PMCID: PMC6894399
- DOI: 10.1016/j.gde.2017.03.002
The role of metabolic states in development and disease
Abstract
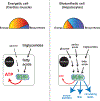
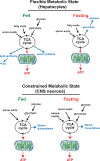
During development, cells adopt distinct metabolic strategies to support growth, produce energy, and meet the demands of a mature tissue. Some of these metabolic states maintain a constrained program of nutrient utilization, while others providing metabolic flexibility as a means to couple developmental progression with nutrient availability. Here we discuss our understanding of metabolic programs, and how they support specific aspects of animal development. During phases of rapid proliferation a subset of metabolic programs provide the building blocks to support growth. During differentiation, metabolic programs shift to support the unique demands of each tissue. Finally, we discuss how a model system, such as Drosophila egg development, can provide a versatile platform to discover novel mechanisms controlling programmed shift in metabolism.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
-
- Tennessen JM, et al., The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab, 2011. 13(2): p. 139–48. - PMC - PubMed
-
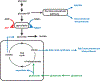
Using a combination of metabolomics and gene expression analysis the authors of this study describe a pronounced shift in metabolism during mid-embryogenesis that favors high rates of glycolysis. This shift to aerobic glycolysis functions to support rapid growth during early Drosophila development.
-
- Sieber MH, Thomsen MB, and Spradling AC, Electron Transport Chain Remodeling by GSK3 during Oogenesis Connects Nutrient State to Reproduction. Cell, 2016. 164(3): p. 420–32. - PMC - PubMed
-
In the final stages of oogenesis oocytes display a very low level of mitochondrial activity. In this study the authors show that establish a form of mitochondrial respiratory quiescence through a pronounce shift in mitochondrial proteins that leads to remodeling of the electron transport chain (ETC). Interestingly, this remodeling of the ETC it regulated in a nutrient-dependent manner by the insulin/AKT signaling pathway which couple systemic metabolism in the germline.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

