WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals
- PMID: 28347989
- PMCID: PMC5450310
- DOI: 10.1242/bio.021717
WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals
Abstract
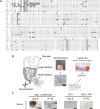
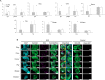
Recent years have seen significant developments in the ability to continuously propagate organoids derived from intestinal crypts. These advancements have been applied to mouse and human samples providing models for gastrointestinal tissue development and disease. We adapt these methods for the propagation of intestinal organoids (enteroids) from various large farm and small companion (LF/SC) animals, including cat, dog, cow, horse, pig, sheep and chicken. We show that LF/SC enteroids propagate and expand in L-WRN conditioned media containing signaling factors Wnt3a, R-spondin-3, and Noggin (WRN). Multiple successful isolations were achieved for each species, and the growth of LF/SC enteroids was maintained to high passage number. LF/SC enteroids expressed crypt stem cell marker LGR5 and low levels of mesenchymal marker VIM. Labeling with EdU also showed distinct regions of cell proliferation within the enteroids marking crypt-like regions. The ability to grow and maintain LF/SC enteroid cell lines provides additional models for the study of gastrointestinal developmental biology as well as platforms for the study of host-pathogen interactions between intestinal cells and zoonotic enteric pathogens of medical importance.
Keywords: Companion animal; Conditioned media; Crypt; Enteroid; Farm animal; Intestine; Organoid.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Aoki R., Shoshkes-Carmel M., Gao N., Shin S., May C. L., Golson M. L., Zahm A. M., Ray M., Wiser C. L., Wright C. V. E. et al. (2016). Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol. Gastroenterol. Hepatol. 2, 175-188. 10.1016/j.jcmgh.2015.12.004 - DOI - PMC - PubMed
-
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H. J., van Es J. H., Sato T., Stange D. E., Begthel H., van den Born M. et al. (2010). Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25-36. 10.1016/j.stem.2009.11.013 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

