TGF-β Signaling in Control of Cardiovascular Function
- PMID: 28348036
- PMCID: PMC5793760
- DOI: 10.1101/cshperspect.a022210
TGF-β Signaling in Control of Cardiovascular Function
Abstract
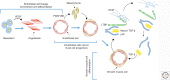
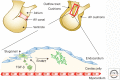
Genetic studies in animals and humans indicate that gene mutations that functionally perturb transforming growth factor β (TGF-β) signaling are linked to specific hereditary vascular syndromes, including Osler-Rendu-Weber disease or hereditary hemorrhagic telangiectasia and Marfan syndrome. Disturbed TGF-β signaling can also cause nonhereditary disorders like atherosclerosis and cardiac fibrosis. Accordingly, cell culture studies using endothelial cells or smooth muscle cells (SMCs), cultured alone or together in two- or three-dimensional cell culture assays, on plastic or embedded in matrix, have shown that TGF-β has a pivotal effect on endothelial and SMC proliferation, differentiation, migration, tube formation, and sprouting. Moreover, TGF-β can stimulate endothelial-to-mesenchymal transition, a process shown to be of key importance in heart valve cushion formation and in various pathological vascular processes. Here, we discuss the roles of TGF-β in vasculogenesis, angiogenesis, and lymphangiogenesis and the deregulation of TGF-β signaling in cardiovascular diseases.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
