Iron Efflux by PmtA Is Critical for Oxidative Stress Resistance and Contributes Significantly to Group A Streptococcus Virulence
- PMID: 28348051
- PMCID: PMC5442632
- DOI: 10.1128/IAI.00091-17
Iron Efflux by PmtA Is Critical for Oxidative Stress Resistance and Contributes Significantly to Group A Streptococcus Virulence
Abstract
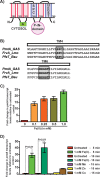
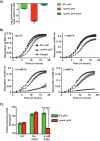
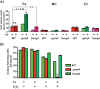
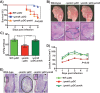
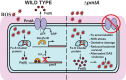
Group A Streptococcus (GAS) is a human-only pathogen that causes a spectrum of disease conditions. Given its survival in inflamed lesions, the ability to sense and overcome oxidative stress is critical for GAS pathogenesis. PerR senses oxidative stress and coordinates the regulation of genes involved in GAS antioxidant defenses. In this study, we investigated the role of PerR-controlled metal transporter A (PmtA) in GAS pathogenesis. Previously, PmtA was implicated in GAS antioxidant defenses and suggested to protect against zinc toxicity. Here, we report that PmtA is a P1B4-type ATPase that functions as an Fe(II) exporter and aids GAS defenses against iron intoxication and oxidative stress. The expression of pmtA is specifically induced by excess iron, and this induction requires PerR. Furthermore, a pmtA mutant exhibited increased sensitivity to iron toxicity and oxidative stress due to an elevated intracellular accumulation of iron. RNA-sequencing analysis revealed that GAS undergoes significant alterations in gene expression to adapt to iron toxicity. Finally, using two mouse models of invasive infection, we demonstrated that iron efflux by PmtA is critical for bacterial survival during infection and GAS virulence. Together, these data demonstrate that PmtA is a key component of GAS antioxidant defenses and contributes significantly to GAS virulence.
Keywords: bacterial pathogenesis; gene regulation; iron efflux; metal homeostasis; oxidative stress.
Copyright © 2017 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

