The herpes simplex virus 1 UL36USP deubiquitinase suppresses DNA repair in host cells via deubiquitination of proliferating cell nuclear antigen
- PMID: 28348081
- PMCID: PMC5437251
- DOI: 10.1074/jbc.M117.778076
The herpes simplex virus 1 UL36USP deubiquitinase suppresses DNA repair in host cells via deubiquitination of proliferating cell nuclear antigen
Abstract
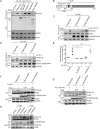
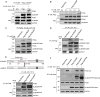
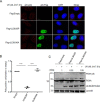
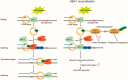
Herpes simplex virus 1 (HSV-1) infection manipulates distinct host DNA-damage responses to facilitate virus proliferation, but the molecular mechanisms remain to be elucidated. One possible HSV-1 target might be DNA damage-tolerance mechanisms, such as the translesion synthesis (TLS) pathway. In TLS, proliferating cell nuclear antigen (PCNA) is monoubiquitinated in response to DNA damage-caused replication fork stalling. Ubiquitinated PCNA then facilitates the error-prone DNA polymerase η (polη)-mediated TLS, allowing the fork to bypass damaged sites. Because of the involvement of PCNA ubiquitination in DNA-damage repair, we hypothesized that the function of PCNA might be altered by HSV-1. Here we show that PCNA is a substrate of the HSV-1 deubiquitinase UL36USP, which has previously been shown to be involved mainly in virus uptake and maturation. In HSV-1-infected cells, viral infection-associated UL36USP consistently reduced PCNA ubiquitination. The deubiquitination of PCNA inhibited the formation of polη foci and also increased cell sensitivity to DNA-damage agents. Moreover, the catalytically inactive mutant UL36C40A failed to deubiquitinate PCNA. Of note, the levels of virus marker genes increased strikingly in cells infected with wild-type HSV-1, but only moderately in UL36C40A mutant virus-infected cells, indicating that the UL36USP deubiquitinating activity supports HSV-1 virus replication during infection. These findings suggest a role of UL36USP in the DNA damage-response pathway.
Keywords: DNA damage response; DNA polymerase; UL36USP; deubiquitylation (deubiquitination); herpesvirus; proliferating cell nuclear antigen (PCNA); translesion synthesis.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Epstein-Barr virus BPLF1 deubiquitinates PCNA and attenuates polymerase η recruitment to DNA damage sites.J Virol. 2012 Aug;86(15):8097-106. doi: 10.1128/JVI.00588-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623772 Free PMC article.
-
The Translesion Polymerase Pol η Is Required for Efficient Epstein-Barr Virus Infectivity and Is Regulated by the Viral Deubiquitinating Enzyme BPLF1.J Virol. 2017 Sep 12;91(19):e00600-17. doi: 10.1128/JVI.00600-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724765 Free PMC article.
-
Herpes Simplex Virus 1 Ubiquitin-Specific Protease UL36 Abrogates NF-κB Activation in DNA Sensing Signal Pathway.J Virol. 2017 Feb 14;91(5):e02417-16. doi: 10.1128/JVI.02417-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28031360 Free PMC article.
-
Spatiotemporal regulation of PCNA ubiquitination in damage tolerance pathways.Crit Rev Biochem Mol Biol. 2019 Oct;54(5):418-442. doi: 10.1080/10409238.2019.1687420. Epub 2019 Nov 18. Crit Rev Biochem Mol Biol. 2019. PMID: 31736364 Review.
-
Ubiquitination of PCNA and its essential role in eukaryotic translesion synthesis.Cell Biochem Biophys. 2011 Jun;60(1-2):47-60. doi: 10.1007/s12013-011-9187-3. Cell Biochem Biophys. 2011. PMID: 21461937 Review.
Cited by
-
Epstein-Barr Virus Early Protein BFRF1 Suppresses IFN-β Activity by Inhibiting the Activation of IRF3.Front Immunol. 2020 Dec 17;11:513383. doi: 10.3389/fimmu.2020.513383. eCollection 2020. Front Immunol. 2020. PMID: 33391252 Free PMC article.
-
Comparison of the Transcriptomes and Proteomes of Serum Exosomes from Marek's Disease Virus-Vaccinated and Protected and Lymphoma-Bearing Chickens.Genes (Basel). 2019 Feb 5;10(2):116. doi: 10.3390/genes10020116. Genes (Basel). 2019. PMID: 30764491 Free PMC article.
-
Herpes simplex virus type I glycoprotein L evades host antiviral innate immunity by abrogating the nuclear translocation of phosphorylated NF-κB sub-unit p65.Front Microbiol. 2023 May 9;14:1178249. doi: 10.3389/fmicb.2023.1178249. eCollection 2023. Front Microbiol. 2023. PMID: 37228366 Free PMC article.
-
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1.Microbiol Mol Biol Rev. 2020 Sep 30;84(4):e00099-20. doi: 10.1128/MMBR.00099-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 32998978 Free PMC article. Review.
-
Herpes Simplex Virus 1 UL2 Inhibits the TNF-α-Mediated NF-κB Activity by Interacting With p65/p50.Front Immunol. 2020 May 13;11:549. doi: 10.3389/fimmu.2020.00549. eCollection 2020. Front Immunol. 2020. PMID: 32477319 Free PMC article.
References
-
- Luftig M. A. (2014) Viruses and the DNA damage response: activation and antagonism. Annu. Rev. Virol. 1, 605–625 - PubMed
-
- Kannouche P. L., Wing J., and Lehmann A. R. (2004) Interaction of human DNA polymerase η with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 14, 491–500 - PubMed
-
- Chapman J. R., Taylor M. R., and Boulton S. J. (2012) Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 47, 497–510 - PubMed
-
- Mailand N., Gibbs-Seymour I., and Bekker-Jensen S. (2013) Regulation of PCNA-protein interactions for genome stability. Nat. Rev. Mol. Cell Biol. 14, 269–282 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

