Rate Coding and the Control of Muscle Force
- PMID: 28348173
- PMCID: PMC5629984
- DOI: 10.1101/cshperspect.a029702
Rate Coding and the Control of Muscle Force
Abstract
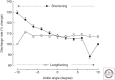
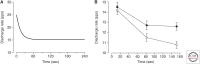
The force exerted by a muscle during a voluntary contraction depends on the number of motor units recruited for the action and the rates at which they discharge action potentials (rate coding). Over most of the operating range of a muscle, the nervous system controls muscle force by varying both motor unit recruitment and rate coding. Except at relatively low forces, however, the control of muscle force depends primarily on rate coding, especially during fast contractions. This review provides five examples of how the modulation of rate coding influences the force exerted by muscle during voluntary actions. The five examples comprise fast contractions, lengthening and shortening contractions, steady isometric contractions, fatiguing contractions, and contractions performed after a change in the daily level of physical activity.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Almuklass A, Davis L, Vieira T, Botter A, Derqaoui K, Hamilton L, Hebert JR, Vollmer TL, Alvarez E, Enoka RM. 2016a. Neuromuscular determinants for walking performance in individuals with multiple sclerosis. Med Sci Sports Exerc 48: 712.
-
- Almuklass AM, Price RC, Gould JR, Enoka RM. 2016b. Force steadiness as a predictor of time to complete a pegboard test of dexterity in young men and women. J Appl Physiol 120: 1410–1417. - PubMed
-
- Altenburg TM, de Ruiter CJ, Verdijk PW, van Mechelen W, de Haan A. 2009. Vastus lateralis surface and single motor unit electromyography during shortening, lengthening and isometric contractions corrected for mode-dependent differences in force-generating capacity. Acta Physiol (Oxford) 196: 315–328. - PubMed
-
- Bailey EF, Rice AD, Fuglevand AJ. 2007. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 97: 933–936. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
