ERRα induces H3K9 demethylation by LSD1 to promote cell invasion
- PMID: 28348226
- PMCID: PMC5393192
- DOI: 10.1073/pnas.1614664114
ERRα induces H3K9 demethylation by LSD1 to promote cell invasion
Abstract
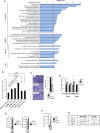
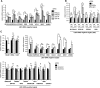
Lysine Specific Demethylase 1 (LSD1) removes mono- and dimethyl groups from lysine 4 of histone H3 (H3K4) or H3K9, resulting in repressive or activating (respectively) transcriptional histone marks. The mechanisms that control the balance between these two antagonist activities are not understood. We here show that LSD1 and the orphan nuclear receptor estrogen-related receptor α (ERRα) display commonly activated genes. Transcriptional activation by LSD1 and ERRα involves H3K9 demethylation at the transcriptional start site (TSS). Strikingly, ERRα is sufficient to induce LSD1 to demethylate H3K9 in vitro. The relevance of this mechanism is highlighted by functional data. LSD1 and ERRα coregulate several target genes involved in cell migration, including the MMP1 matrix metallo-protease, also activated through H3K9 demethylation at the TSS. Depletion of LSD1 or ERRα reduces the cellular capacity to invade the extracellular matrix, a phenomenon that is rescued by MMP1 reexpression. Altogether our results identify a regulatory network involving a direct switch in the biochemical activities of a histone demethylase, leading to increased cell invasion.
Keywords: ERRα; LSD1; cell migration; histone demethylation; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


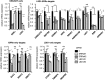
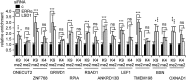


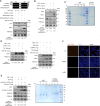
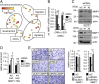
References
-
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128(4):707–719. - PubMed
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. - PubMed
-
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: A coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20(11):1405–1428. - PubMed
-
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. - PubMed
-
- Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25(1):1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

