Structural basis of mitochondrial dysfunction in response to cytochrome c phosphorylation at tyrosine 48
- PMID: 28348229
- PMCID: PMC5393209
- DOI: 10.1073/pnas.1618008114
Structural basis of mitochondrial dysfunction in response to cytochrome c phosphorylation at tyrosine 48
Abstract
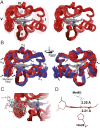
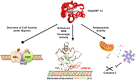
Regulation of mitochondrial activity allows cells to adapt to changing conditions and to control oxidative stress, and its dysfunction can lead to hypoxia-dependent pathologies such as ischemia and cancer. Although cytochrome c phosphorylation-in particular, at tyrosine 48-is a key modulator of mitochondrial signaling, its action and molecular basis remain unknown. Here we mimic phosphorylation of cytochrome c by replacing tyrosine 48 with p-carboxy-methyl-l-phenylalanine (pCMF). The NMR structure of the resulting mutant reveals significant conformational shifts and enhanced dynamics around pCMF that could explain changes observed in its functionality: The phosphomimetic mutation impairs cytochrome c diffusion between respiratory complexes, enhances hemeprotein peroxidase and reactive oxygen species scavenging activities, and hinders caspase-dependent apoptosis. Our findings provide a framework to further investigate the modulation of mitochondrial activity by phosphorylated cytochrome c and to develop novel therapeutic approaches based on its prosurvival effects.
Keywords: cytochrome c; mitochondrial dysfunction; nuclear magnetic resonance; phosphorylation; respiratory supercomplexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria.Proc Natl Acad Sci U S A. 2018 Jul 31;115(31):7955-7960. doi: 10.1073/pnas.1806833115. Epub 2018 Jul 17. Proc Natl Acad Sci U S A. 2018. PMID: 30018060 Free PMC article.
-
Serine-47 phosphorylation of cytochrome c in the mammalian brain regulates cytochrome c oxidase and caspase-3 activity.FASEB J. 2019 Dec;33(12):13503-13514. doi: 10.1096/fj.201901120R. Epub 2019 Sep 28. FASEB J. 2019. PMID: 31570002 Free PMC article.
-
Tyrosine phosphorylation turns alkaline transition into a biologically relevant process and makes human cytochrome c behave as an anti-apoptotic switch.J Biol Inorg Chem. 2011 Dec;16(8):1155-68. doi: 10.1007/s00775-011-0804-9. Epub 2011 Jun 25. J Biol Inorg Chem. 2011. PMID: 21706253
-
Pathophysiological implications of mitochondrial oxidative stress mediated by mitochondriotropic agents and polyamines: the role of tyrosine phosphorylation.Amino Acids. 2015 May;47(5):869-83. doi: 10.1007/s00726-015-1964-7. Epub 2015 Mar 20. Amino Acids. 2015. PMID: 25792113 Review.
-
Free radical oxidation of cardiolipin: chemical mechanisms, detection and implication in apoptosis, mitochondrial dysfunction and human diseases.Free Radic Res. 2012 Aug;46(8):959-74. doi: 10.3109/10715762.2012.676642. Epub 2012 Apr 10. Free Radic Res. 2012. PMID: 22468920 Review.
Cited by
-
Streamlined Postprocessing of NMR Structures with the Molecular Restrainer: A Universal Tool for High-Quality Protein-Ligand Models and Non-Standard Amino Acid Residues.Int J Mol Sci. 2025 May 26;26(11):5091. doi: 10.3390/ijms26115091. Int J Mol Sci. 2025. PMID: 40507902 Free PMC article.
-
Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis.Int J Biochem Cell Biol. 2020 Apr;121:105704. doi: 10.1016/j.biocel.2020.105704. Epub 2020 Feb 2. Int J Biochem Cell Biol. 2020. PMID: 32023432 Free PMC article.
-
Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration.Sci Rep. 2017 Nov 13;7(1):15424. doi: 10.1038/s41598-017-14970-0. Sci Rep. 2017. PMID: 29133913 Free PMC article.
-
Genetic Encoding of Phosphorylated Amino Acids into Proteins.Chem Rev. 2024 May 22;124(10):6592-6642. doi: 10.1021/acs.chemrev.4c00110. Epub 2024 May 1. Chem Rev. 2024. PMID: 38691379 Free PMC article. Review.
-
Exploring protein phosphorylation by combining computational approaches and biochemical methods.Comput Struct Biotechnol J. 2020 Jul 7;18:1852-1863. doi: 10.1016/j.csbj.2020.06.043. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32728408 Free PMC article.
References
-
- Papa S. Molecular mechanism of proton translocation by the cytochrome system and the ATPase of mitochondria. Role of proteins. J Bioenerg Biomembr. 1982;14:69–86. - PubMed
-
- Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: A new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

