Mechanical design in embryos: mechanical signalling, robustness and developmental defects
- PMID: 28348252
- PMCID: PMC5379024
- DOI: 10.1098/rstb.2015.0516
Mechanical design in embryos: mechanical signalling, robustness and developmental defects
Abstract
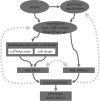
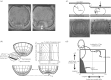
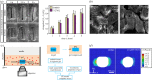
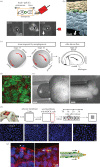
Embryos are shaped by the precise application of force against the resistant structures of multicellular tissues. Forces may be generated, guided and resisted by cells, extracellular matrix, interstitial fluids, and how they are organized and bound within the tissue's architecture. In this review, we summarize our current thoughts on the multiple roles of mechanics in direct shaping, mechanical signalling and robustness of development. Genetic programmes of development interact with environmental cues to direct the composition of the early embryo and endow cells with active force production. Biophysical advances now provide experimental tools to measure mechanical resistance and collective forces during morphogenesis and are allowing integration of this field with studies of signalling and patterning during development. We focus this review on concepts that highlight this integration, and how the unique contributions of mechanical cues and gradients might be tested side by side with conventional signalling systems. We conclude with speculation on the integration of large-scale programmes of development, and how mechanical responses may ensure robust development and serve as constraints on programmes of tissue self-assembly.This article is part of the themed issue 'Systems morphodynamics: understanding the development of tissue hardware'.
Keywords: biomechanics; developmental defect; mechanobiology; morphogenesis; organogenesis; self-assembly.
© 2017 The Author(s).
Figures
References
-
- His W. 1888. On the principles of animal morphology. Proc. R. Soc. Edinb. 15, 287–298.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
