Endocannabinoid signalling modulates susceptibility to traumatic stress exposure
- PMID: 28348378
- PMCID: PMC5379055
- DOI: 10.1038/ncomms14782
Endocannabinoid signalling modulates susceptibility to traumatic stress exposure
Abstract
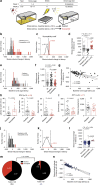
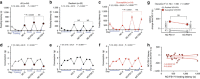
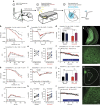
Stress is a ubiquitous risk factor for the exacerbation and development of affective disorders including major depression and posttraumatic stress disorder. Understanding the neurobiological mechanisms conferring resilience to the adverse consequences of stress could have broad implications for the treatment and prevention of mood and anxiety disorders. We utilize laboratory mice and their innate inter-individual differences in stress-susceptibility to demonstrate a critical role for the endogenous cannabinoid 2-arachidonoylglycerol (2-AG) in stress-resilience. Specifically, systemic 2-AG augmentation is associated with a stress-resilient phenotype and enhances resilience in previously susceptible mice, while systemic 2-AG depletion or CB1 receptor blockade increases susceptibility in previously resilient mice. Moreover, stress-resilience is associated with increased phasic 2-AG-mediated synaptic suppression at ventral hippocampal-amygdala glutamatergic synapses and amygdala-specific 2-AG depletion impairs successful adaptation to repeated stress. These data indicate amygdala 2-AG signalling mechanisms promote resilience to adverse effects of acute traumatic stress and facilitate adaptation to repeated stress exposure.
Conflict of interest statement
S.P. and L.M. have an active collaborative research contract with Lundbeck Pharmaceuticals, however, the work presented in the paper was supported solely by the NIH, the Brain & Behavior Research Foundation, and Vanderbilt University.
Figures






References
-
- Faravelli C. Life events preceding the onset of panic disorder. J. Affect. Disord. 9, 103–105 (1985). - PubMed
-
- Kendler K. S., Karkowski L. M. & Prescott C. A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841 (1999). - PubMed
-
- Kessler R. C. et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

