Inhibiting the system xC-/glutathione axis selectively targets cancers with mutant-p53 accumulation
- PMID: 28348409
- PMCID: PMC5379068
- DOI: 10.1038/ncomms14844
Inhibiting the system xC-/glutathione axis selectively targets cancers with mutant-p53 accumulation
Abstract
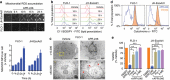
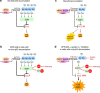
TP53, a critical tumour suppressor gene, is mutated in over half of all cancers resulting in mutant-p53 protein accumulation and poor patient survival. Therapeutic strategies to target mutant-p53 cancers are urgently needed. We show that accumulated mutant-p53 protein suppresses the expression of SLC7A11, a component of the cystine/glutamate antiporter, system xC-, through binding to the master antioxidant transcription factor NRF2. This diminishes glutathione synthesis, rendering mutant-p53 tumours susceptible to oxidative damage. System xC- inhibitors specifically exploit this vulnerability to preferentially kill cancer cells with stabilized mutant-p53 protein. Moreover, we demonstrate that SLC7A11 expression is a novel and robust predictive biomarker for APR-246, a first-in-class mutant-p53 reactivator that also binds and depletes glutathione in tumours, triggering lipid peroxidative cell death. Importantly, system xC- antagonism strongly synergizes with APR-246 to induce apoptosis in mutant-p53 tumours. We propose a new paradigm for targeting cancers that accumulate mutant-p53 protein by inhibiting the SLC7A11-glutathione axis.
Conflict of interest statement
K.G.W. is a cofounder and shareholder of Aprea Therapeutics, and L.A. is the chief scientific officer for Aprea Therapeutics, a company that develops p53-based anticancer therapy including APR-246. All other authors declare no competing financial interests.
Figures







References
-
- Muller P. A. & Vousden K. H. p53 mutations in cancer. Nat. Cell Biol. 15, 2–8 (2013). - PubMed
-
- Bykov V. J. & Wiman K. G. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett. 588, 2622–2627 (2014). - PubMed
-
- Liu D. S. et al. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma. Gut 64, 1506–1516 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

