Exploring the genomic diversity of black yeasts and relatives (Chaetothyriales, Ascomycota)
- PMID: 28348446
- PMCID: PMC5358931
- DOI: 10.1016/j.simyco.2017.01.001
Exploring the genomic diversity of black yeasts and relatives (Chaetothyriales, Ascomycota)
Abstract
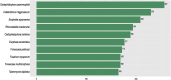
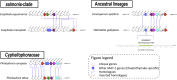
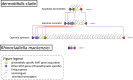
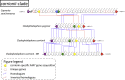
The order Chaetothyriales (Pezizomycotina, Ascomycetes) harbours obligatorily melanised fungi and includes numerous etiologic agents of chromoblastomycosis, phaeohyphomycosis and other diseases of vertebrate hosts. Diseases range from mild cutaneous to fatal cerebral or disseminated infections and affect humans and cold-blooded animals globally. In addition, Chaetothyriales comprise species with aquatic, rock-inhabiting, ant-associated, and mycoparasitic life-styles, as well as species that tolerate toxic compounds, suggesting a high degree of versatile extremotolerance. To understand their biology and divergent niche occupation, we sequenced and annotated a set of 23 genomes of main the human opportunists within the Chaetothyriales as well as related environmental species. Our analyses included fungi with diverse life-styles, namely opportunistic pathogens and closely related saprobes, to identify genomic adaptations related to pathogenesis. Furthermore, ecological preferences of Chaetothyriales were analysed, in conjuncture with the order-level phylogeny based on conserved ribosomal genes. General characteristics, phylogenomic relationships, transposable elements, sex-related genes, protein family evolution, genes related to protein degradation (MEROPS), carbohydrate-active enzymes (CAZymes), melanin synthesis and secondary metabolism were investigated and compared between species. Genome assemblies varied from 25.81 Mb (Capronia coronata) to 43.03 Mb (Cladophialophora immunda). The bantiana-clade contained the highest number of predicted genes (12 817 on average) as well as larger genomes. We found a low content of mobile elements, with DNA transposons from Tc1/Mariner superfamily being the most abundant across analysed species. Additionally, we identified a reduction of carbohydrate degrading enzymes, specifically many of the Glycosyl Hydrolase (GH) class, while most of the Pectin Lyase (PL) genes were lost in etiological agents of chromoblastomycosis and phaeohyphomycosis. An expansion was found in protein degrading peptidase enzyme families S12 (serine-type D-Ala-D-Ala carboxypeptidases) and M38 (isoaspartyl dipeptidases). Based on genomic information, a wide range of abilities of melanin biosynthesis was revealed; genes related to metabolically distinct DHN, DOPA and pyomelanin pathways were identified. The MAT (MAting Type) locus and other sex-related genes were recognized in all 23 black fungi. Members of the asexual genera Fonsecaea and Cladophialophora appear to be heterothallic with a single copy of either MAT-1-1 or MAT-1-2 in each individual. All Capronia species are homothallic as both MAT1-1 and MAT1-2 genes were found in each single genome. The genomic synteny of the MAT-locus flanking genes (SLA2-APN2-COX13) is not conserved in black fungi as is commonly observed in Eurotiomycetes, indicating a unique genomic context for MAT in those species. The heterokaryon (het) genes expansion associated with the low selective pressure at the MAT-locus suggests that a parasexual cycle may play an important role in generating diversity among those fungi.
Keywords: Black yeast; Chaetothyriales; Comparative genomics; Ecology; Evolution; Herpotrichiellaceae; Phylogeny.
Figures








References
-
- Alviano C.S., Farbiarz S.R., De Souza W. Characterization of Fonsecaea pedrosoi melanin. Journal of General Microbiology. 1991;137:837–844. - PubMed
-
- Aptroot A., Diederich P., Sérusiaux E. Lichens and lichenicolous fungi. Bibliotheca Lichenologica. 1997;64:1–220.
-
- Attygalle A.B., Siegel B., Vostrowsky O. Chemical composition and function of metapleural gland secretion of the ant, Crematogaster deformis smith (Hymenoptera: Myrmicinae) Journal of Chemical Ecology. 1989;15:317–328. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
