Lack of β2-AR Increases Anxiety-Like Behaviors and Rewarding Properties of Cocaine
- PMID: 28348522
- PMCID: PMC5346996
- DOI: 10.3389/fnbeh.2017.00049
Lack of β2-AR Increases Anxiety-Like Behaviors and Rewarding Properties of Cocaine
Abstract
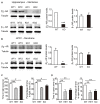
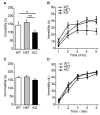
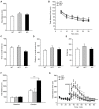
It is well known that β-adrenoceptors (β-ARs) play a critical role in emotional arousal and stressful events, but the specific contributions of the β2-AR subtype to the psychological disorders are largely unknown. To investigate whether β2-AR are involved in anxiety-like behavior and reward to addictive drugs, we conducted a series of behavioral tests on β2-AR knock-out (KO) mice. β2-AR KO mice exhibited increased preference for the dark compartment and closed arm in tests of Light/Dark box and elevated plus maze, indicating that β2-AR deletion elevates level of anxiety or innate fear. β2-AR KO mice also showed decreased immobility in tail suspension test (TST), suggesting that β2-AR deletion inhibits depression-like behavior. Interestingly, β2-AR ablation did not change basal locomotion but significantly increased locomotor activity induced by acute cocaine administration. β2-AR KO mice showed enhanced place preference for cocaine, which could be attenuated by β1-selective AR antagonist betaxolol. Consistently, β2-AR agonist suppressed cocaine-conditioned place preference (CPP). These data indicate that β2-AR deletion enhances acute response and reward to cocaine. Our results suggest that β2-AR regulates anxiety level, depression-like behavior and hedonic properties of cocaine, implicating that β2-AR are the potential targets for the treatment of emotional disorders and cocaine addiction.
Keywords: anxiety; cocaine; depression; reward; β2-AR.
Figures
Similar articles
-
Dissociation of β1- and β2-adrenergic receptor subtypes in the retrieval of cocaine-associated memory.Behav Brain Res. 2016 Jan 1;296:94-99. doi: 10.1016/j.bbr.2015.08.030. Epub 2015 Aug 28. Behav Brain Res. 2016. PMID: 26318933 Free PMC article.
-
Sex differences in hippocampal β-adrenergic receptor subtypes drive retrieval, retention, and learning of cocaine-associated memories.Front Behav Neurosci. 2024 May 14;18:1379866. doi: 10.3389/fnbeh.2024.1379866. eCollection 2024. Front Behav Neurosci. 2024. PMID: 38807929 Free PMC article.
-
Fatty acid binding protein deletion prevents stress-induced preference for cocaine and dampens stress-induced corticosterone levels.Synapse. 2018 Jun;72(6):e22031. doi: 10.1002/syn.22031. Epub 2018 Feb 19. Synapse. 2018. PMID: 29457656 Free PMC article.
-
β-Adrenoceptor-mediated Relaxation of Urinary Bladder Muscle in β2-Adrenoceptor Knockout Mice.Front Pharmacol. 2016 May 9;7:118. doi: 10.3389/fphar.2016.00118. eCollection 2016. Front Pharmacol. 2016. PMID: 27242525 Free PMC article.
-
Molecular mechanisms underlying the rewarding effects of cocaine.Ann N Y Acad Sci. 2004 Oct;1025:47-56. doi: 10.1196/annals.1316.006. Ann N Y Acad Sci. 2004. PMID: 15542699 Review.
Cited by
-
Anxiolytic Effect of Carvedilol in Chronic Unpredictable Stress Model.Oxid Med Cell Longev. 2022 Aug 19;2022:6906722. doi: 10.1155/2022/6906722. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36035219 Free PMC article.
-
β2-Adrenoceptors in the Medial Prefrontal Cortex Excitatory Neurons Regulate Anxiety-like Behavior in Mice.Int J Mol Sci. 2022 May 17;23(10):5578. doi: 10.3390/ijms23105578. Int J Mol Sci. 2022. PMID: 35628393 Free PMC article.
-
Repeated vagus nerve stimulation produces anxiolytic effects via upregulation of AMPAR function in centrolateral amygdala of male rats.Neurobiol Stress. 2022 Apr 22;18:100453. doi: 10.1016/j.ynstr.2022.100453. eCollection 2022 May. Neurobiol Stress. 2022. PMID: 35685681 Free PMC article.
-
Heart rate response to cognitive load as a marker of depression and increased anxiety.Front Psychiatry. 2024 Jul 11;15:1355846. doi: 10.3389/fpsyt.2024.1355846. eCollection 2024. Front Psychiatry. 2024. PMID: 39056018 Free PMC article.
References
-
- Balasubramaniyan N., Rayapati D. K., Puttiah R. H., Tavane P., Singh S. E., Rangan V., et al. . (2016). Evaluation of anxiety induced cardiovascular response in known hypertensive patients undergoing exodontia—a prospective study. J. Clin. Diagn. Res. 10, ZC123–ZC127. 10.7860/JCDR/2016/19685.8391 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

