Mitochondrial Bioenergetics in the Metabolic Myopathy Accompanying Peripheral Artery Disease
- PMID: 28348531
- PMCID: PMC5346567
- DOI: 10.3389/fphys.2017.00141
Mitochondrial Bioenergetics in the Metabolic Myopathy Accompanying Peripheral Artery Disease
Abstract
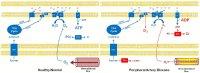
Peripheral artery disease (PAD) is a serious but relatively underdiagnosed and undertreated clinical condition associated with a marked reduction in functional capacity and a heightened risk of morbidity and mortality. The pathophysiology of lower extremity PAD is complex, and extends beyond the atherosclerotic arterial occlusion and subsequent mismatch between oxygen demand and delivery to skeletal muscle mitochondria. In this review, we evaluate and summarize the available evidence implicating mitochondria in the metabolic myopathy that accompanies PAD. Following a short discussion of the available in vivo and in vitro methodologies to quantitate indices of muscle mitochondrial function, we review the current evidence implicating skeletal muscle mitochondrial dysfunction in the pathophysiology of PAD myopathy, while attempting to highlight questions that remain unanswered. Given the rising prevalence of PAD, the detriment in quality of life for patients, and the associated significant healthcare resource utilization, new alternate therapies that ameliorate lower limb symptoms and the functional impairment associated with PAD are needed. A clear understanding of the role of mitochondria in the pathophysiology of PAD may contribute to the development of novel therapeutic interventions.
Keywords: bioenergetics; mitochondria; mitochondrial function; peripheral artery disease; peripheral vascular disease; skeletal muscle.
Figures
Similar articles
-
Ultrastructural alterations and mitochondrial dysfunction in skeletal muscle of peripheral artery disease patients: implications for early therapeutic interventions.EXCLI J. 2024 Oct 7;23:1208-1225. doi: 10.17179/excli2024-7592. eCollection 2024. EXCLI J. 2024. PMID: 39574966 Free PMC article.
-
Impaired microcirculatory function, mitochondrial respiration, and oxygen utilization in skeletal muscle of claudicating patients with peripheral artery disease.Am J Physiol Heart Circ Physiol. 2022 May 1;322(5):H867-H879. doi: 10.1152/ajpheart.00690.2021. Epub 2022 Mar 25. Am J Physiol Heart Circ Physiol. 2022. PMID: 35333113 Free PMC article.
-
Increased skeletal muscle mitochondrial free radical production in peripheral arterial disease despite preserved mitochondrial respiratory capacity.Exp Physiol. 2018 Jun;103(6):838-850. doi: 10.1113/EP086905. Epub 2018 May 8. Exp Physiol. 2018. PMID: 29604234 Free PMC article.
-
Disruption of mitochondrial quality control in peripheral artery disease: New therapeutic opportunities.Pharmacol Res. 2017 Jan;115:96-106. doi: 10.1016/j.phrs.2016.11.016. Epub 2016 Nov 19. Pharmacol Res. 2017. PMID: 27876411 Free PMC article. Review.
-
Skeletal Muscle Mitochondrial Dysfunction and Oxidative Stress in Peripheral Arterial Disease: A Unifying Mechanism and Therapeutic Target.Antioxidants (Basel). 2020 Dec 18;9(12):1304. doi: 10.3390/antiox9121304. Antioxidants (Basel). 2020. PMID: 33353218 Free PMC article. Review.
Cited by
-
Mitochondria-Targeted Drugs.Curr Mol Pharmacol. 2019;12(3):202-214. doi: 10.2174/1874467212666181127151059. Curr Mol Pharmacol. 2019. PMID: 30479224 Free PMC article. Review.
-
Gait pattern in patients with peripheral artery disease.BMC Geriatr. 2018 Feb 20;18(1):52. doi: 10.1186/s12877-018-0727-1. BMC Geriatr. 2018. PMID: 29458330 Free PMC article.
-
Mitochondrial Implications in Cardiovascular Aging and Diseases: The Specific Role of Mitochondrial Dynamics and Shifts.Int J Mol Sci. 2022 Mar 9;23(6):2951. doi: 10.3390/ijms23062951. Int J Mol Sci. 2022. PMID: 35328371 Free PMC article. Review.
-
Skeletal Muscle Mitochondrial Protein Synthesis and Respiration Increase With Low-Load Blood Flow Restricted as Well as High-Load Resistance Training.Front Physiol. 2018 Dec 17;9:1796. doi: 10.3389/fphys.2018.01796. eCollection 2018. Front Physiol. 2018. PMID: 30618808 Free PMC article.
-
Dynamic Multiscale Regulation of Perfusion Recovery in Experimental Peripheral Arterial Disease: A Mechanistic Computational Model.JACC Basic Transl Sci. 2022 Jan 5;7(1):28-50. doi: 10.1016/j.jacbts.2021.10.014. eCollection 2022 Jan. JACC Basic Transl Sci. 2022. PMID: 35128207 Free PMC article.
References
-
- Anderson J. D., Epstein F. H., Meyer C. H., Hagspiel K. D., Wang H., Berr S. S., et al. . (2009). Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J. Am. Coll. Cardiol. 54, 628–635. 10.1016/j.jacc.2009.01.080 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

