Dementia with Lewy Bodies: Molecular Pathology in the Frontal Cortex in Typical and Rapidly Progressive Forms
- PMID: 28348546
- PMCID: PMC5346561
- DOI: 10.3389/fneur.2017.00089
Dementia with Lewy Bodies: Molecular Pathology in the Frontal Cortex in Typical and Rapidly Progressive Forms
Abstract
Objectives: The goal of this study was to assess mitochondrial function, energy, and purine metabolism, protein synthesis machinery from the nucleolus to the ribosome, inflammation, and expression of newly identified ectopic olfactory receptors (ORs) and taste receptors (TASRs) in the frontal cortex of typical cases of dementia with Lewy bodies (DLB) and cases with rapid clinical course (rpDLB: 2 years or less) compared with middle-aged non-affected individuals, in order to learn about the biochemical abnormalities underlying Lewy body pathology.
Methods: Real-time quantitative PCR, mitochondrial enzymatic assays, and analysis of β-amyloid, tau, and synuclein species were used.
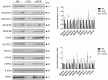
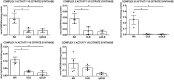
Results: The main alterations in DLB and rpDLB, which are more marked in the rapidly progressive forms, include (i) deregulated expression of several mRNAs and proteins of mitochondrial subunits, and reduced activity of complexes I, II, III, and IV of the mitochondrial respiratory chain; (ii) reduced expression of selected molecules involved in energy metabolism and increased expression of enzymes involved in purine metabolism; (iii) abnormal expression of nucleolar proteins, rRNA18S, genes encoding ribosomal proteins, and initiation factors of the transcription at the ribosome; (iv) discrete inflammation; and (v) marked deregulation of brain ORs and TASRs, respectively. Severe mitochondrial dysfunction involving activity of four complexes, minimal inflammatory responses, and dramatic altered expression of ORs and TASRs discriminate DLB from Alzheimer's disease. Altered solubility and aggregation of α-synuclein, increased β-amyloid bound to membranes, and absence of soluble tau oligomers are common in DLB and rpDLB. Low levels of soluble β-amyloid are found in DLB. However, increased soluble β-amyloid 1-40 and β-amyloid 1-42, and increased TNFα mRNA and protein expression, distinguish rpDLB.
Conclusion: Molecular alterations in frontal cortex in DLB involve key biochemical pathways such as mitochondria and energy metabolism, protein synthesis, purine metabolism, among others and are accompanied by discrete innate inflammatory response.
Keywords: Alzheimer’s disease; dementia with Lewy bodies; inflammation; mitochondria; olfactory receptors; protein synthesis; α-synuclein; β-amyloid.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

