Barriers to Radiation-Induced In Situ Tumor Vaccination
- PMID: 28348554
- PMCID: PMC5346586
- DOI: 10.3389/fimmu.2017.00229
Barriers to Radiation-Induced In Situ Tumor Vaccination
Abstract
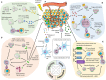
The immunostimulatory properties of radiation therapy (RT) have recently generated widespread interest due to preclinical and clinical evidence that tumor-localized RT can sometimes induce antitumor immune responses mediating regression of non-irradiated metastases (abscopal effect). The ability of RT to activate antitumor T cells explains the synergy of RT with immune checkpoint inhibitors, which has been well documented in mouse tumor models and is supported by observations of more frequent abscopal responses in patients refractory to immunotherapy who receive RT during immunotherapy. However, abscopal responses following RT remain relatively rare in the clinic, and antitumor immune responses are not effectively induced by RT against poorly immunogenic mouse tumors. This suggests that in order to improve the pro-immunogenic effects of RT, it is necessary to identify and overcome the barriers that pre-exist and/or are induced by RT in the tumor microenvironment. On the one hand, RT induces an immunogenic death of cancer cells associated with release of powerful danger signals that are essential to recruit and activate dendritic cells (DCs) and initiate antitumor immune responses. On the other hand, RT can promote the generation of immunosuppressive mediators that hinder DCs activation and impair the function of effector T cells. In this review, we discuss current evidence that several inhibitory pathways are induced and modulated in irradiated tumors. In particular, we will focus on factors that regulate and limit radiation-induced immunogenicity and emphasize current research on actionable targets that could increase the effectiveness of radiation-induced in situ tumor vaccination.
Keywords: abscopal effect; adenosine; hypoxia; immunotherapy; macrophages; radiation therapy; transforming growth factor-β; tumor microenvironment.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

