Temporal Genetic Dynamics of an Experimental, Biparental Field Population of Phytophthora capsici
- PMID: 28348576
- PMCID: PMC5347166
- DOI: 10.3389/fgene.2017.00026
Temporal Genetic Dynamics of an Experimental, Biparental Field Population of Phytophthora capsici
Abstract
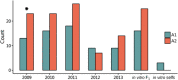
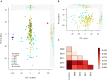
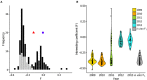
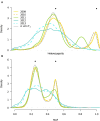
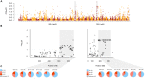
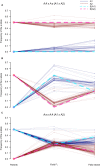
Defining the contributions of dispersal, reproductive mode, and mating system to the population structure of a pathogenic organism is essential to estimating its evolutionary potential. After introduction of the devastating plant pathogen, Phytophthora capsici, into a grower's field, a lack of aerial spore dispersal restricts migration. Once established, coexistence of both mating types results in formation of overwintering recombinant oospores, engendering persistent pathogen populations. To mimic these conditions, in 2008, we inoculated a field with two P. capsici isolates of opposite mating type. We analyzed pathogenic isolates collected in 2009-2013 from this experimental population, using genome-wide single-nucleotide polymorphism markers. By tracking heterozygosity across years, we show that the population underwent a generational shift; transitioning from exclusively F1 in 2009-2010, to multi-generational in 2011, and ultimately all inbred in 2012-2013. Survival of F1 oospores, characterized by heterozygosity excess, coupled with a low rate of selfing, delayed declines in heterozygosity due to inbreeding and attainment of equilibrium genotypic frequencies. Large allele and haplotype frequency changes in specific genomic regions accompanied the generational shift, representing putative signatures of selection. Finally, we identified an approximately 1.6 Mb region associated with mating type determination, constituting the first detailed genomic analysis of a mating type region (MTR) in Phytophthora. Segregation patterns in the MTR exhibited tropes of sex-linkage, where maintenance of allele frequency differences between isolates of opposite mating type was associated with elevated heterozygosity despite inbreeding. Characterizing the trajectory of this experimental system provides key insights into the processes driving persistent, sexual pathogen populations.
Keywords: Phytophthora; bottleneck; inbreeding; mating system; mating type; plant pathogen; population genetics; self-fertilization.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

