Molecular and biochemical characterization of the NS1 protein of non-cultured influenza B virus strains circulating in Singapore
- PMID: 28348873
- PMCID: PMC5320597
- DOI: 10.1099/mgen.0.000082
Molecular and biochemical characterization of the NS1 protein of non-cultured influenza B virus strains circulating in Singapore
Abstract
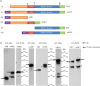
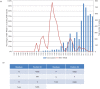
In this study we compared the NS1 protein of Influenza B/Lee/40 and several non-cultured Influenza B virus clinical strains detected in Singapore. In B/Lee/40 virus-infected cells and in cells expressing the recombinant B/Lee/40 NS1 protein a full-length 35 kDa NS1 protein and a 23 kDa NS1 protein species (p23) were detected. Mutational analysis of the NS1 gene indicated that p23 was generated by a novel cleavage event within the linker domain between an aspartic acid and proline at amino acid residues at positions 92 and 93 respectively (DP92-93), and that p23 contained the first 92 amino acids of the NS1 protein. Sequence analysis of the Singapore strains indicated the presence of either DP92-93 or NP92-93 in the NS1 protein, but protein expression analysis showed that p23 was only detected in NS1 proteins with DP92-93.. An additional adjacent proline residue at position 94 (P94) was present in some strains and correlated with increased p23 levels, suggesting that P94 has a synergistic effect on the cleavage of the NS1 protein. The first 145 amino acids of the NS1 protein are required for inhibition of ISG15-mediated ubiquitination, and our analysis showed that Influenza B viruses circulating in Singapore with DP92-93 expressed truncated NS1 proteins and may differ in their capacity to inhibit ISG15 activity. Thus, DP92-93 in the NS1 protein may confer a disadvantage to Influenza B viruses circulating in the human population and interestingly the low frequency of DP92-93detection in the NS1 protein since 2004 is consistent with this suggestion.
Keywords: B/Lee/40; Cleavage; Influenza B; NS1; Proteolysis.
Figures





References
Data Bibliography
-
- Jumat, M. R., Sugrue, R. J. & Tan, B. H., GenBank, Influenza B virus nuclear export protein (NEP) gene, partial cds; and nonstructural protein 1 (NS1) gene, complete cds. PopSet: 482577243 KC844161-KC844196 (2014)
-
- Jumat, M. R., Sugrue, R. J. & Tan, B. H., GenBankGenBank, Influenza B virus (B/Singapore/DSO_090136/2004) segment 8 nuclear export protein (NEP) and nonstructural protein 1 (NS1) genes, complete cds. GenBankGenBank: KC844195.1 (2016)
-
- Jumat, M. R., Sugrue, R. J. & Tan, B. H., GenBankGenBank, Influenza B virus (B/Singapore/DSO_040117Variant/2006) segment 8 NS1 gene, complete sequence; nonstructural protein 1 (NEP) gene, complete cds; and nuclear export protein (NS2) gene, partial cds, GenBankGenBank: KU500812.1 (2016)
-
- Jumat, M. R., Sugrue, R. J. & Tan, B. H., GenBankGenBank, Influenza B virus (B/Singapore/DSO_010147/2007) segment 8 nuclear export protein (NEP) and nonstructural protein 1 (NS1) genes, complete cds, GenBankGenBank: KC844165.1 (2016)
-
- Jumat, M. R., Sugrue, R. J. & Tan, B. H., GenBankGenBank, Influenza B virus (B/Singapore/DSO_020132/2007) segment 8 nuclear export protein (NEP) and nonstructural protein 1 (NS1) genes, complete cds, GenBankGenBank: KC844167.1 (2016)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

