Maxillary Sinus Augmentation with Decellularized Bovine Compact Particles: A Radiological, Clinical, and Histologic Report of 4 Cases
- PMID: 28349056
- PMCID: PMC5352871
- DOI: 10.1155/2017/2594670
Maxillary Sinus Augmentation with Decellularized Bovine Compact Particles: A Radiological, Clinical, and Histologic Report of 4 Cases
Abstract
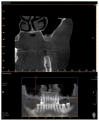
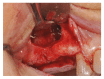
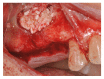
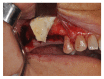
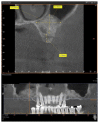
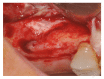
Background. One of the most problematic regions for endosseous implants is the posterior maxilla, not only having poor bone density, but also lacking adequate vertical height as a result of sinus pneumatization. The purpose of the present study was a radiologic, histological, and histomorphometrical evaluation, in humans, of specimens retrieved from sinuses augmented with decellularized bovine compact particles, after a healing period of 6 months. Methods. Four patients, with atrophic resorbed maxillas, underwent a sinus lift augmentation with decellularized bovine compact bone from bovine femur. The size of the particles used was 0.25-1 mm. A total of four grafts and 5 biopsies were retrieved and processed to obtain thin ground sections with the Precise 1 Automated System. Results. The mean volume after graft elevation calculated for each of the 4 patients was 2106 mm3 in the immediate postoperative period (5-7 days), ranging from 1408.8 to 2946.4 mm3. In the late postoperative period (6 months) it was 2053 mm3, ranging from 1339.9 to 2808.9 mm3. Histomorphometry showed that newly formed bone was 36 ± 1.6% and marrow spaces were 34 ± 1.6%, while the residual graft material was 35 ± 1.4%. Conclusion. In conclusion, based on the outcome of the present study, Re-Bone® can be used with success in sinus augmentation procedures and 6 months are considered an adequate time for maturation before implant placement.
Conflict of interest statement
The author declares that there are no competing interests.
Figures



Similar articles
-
Retrospective analysis of three different xenografts in maxillary sinus augmentation: histologic and three-dimensional radiologic study.Quintessence Int. 2023 Sep 19;54(8):640-649. doi: 10.3290/j.qi.b4146671. Quintessence Int. 2023. PMID: 37288651
-
Guided maxillary sinus floor elevation using deproteinized bovine bone versus graftless Schneiderian membrane elevation with simultaneous implant placement: Randomized clinical trial.Clin Implant Dent Relat Res. 2018 Jun;20(3):424-433. doi: 10.1111/cid.12601. Epub 2018 Mar 25. Clin Implant Dent Relat Res. 2018. PMID: 29575547 Clinical Trial.
-
A clinical and histological case series study on calcium sulfate for maxillary sinus floor augmentation and delayed placement of dental implants.Clin Implant Dent Relat Res. 2012 Apr;14(2):259-65. doi: 10.1111/j.1708-8208.2009.00249.x. Epub 2009 Oct 16. Clin Implant Dent Relat Res. 2012. PMID: 19843103
-
Clinical and Histological Outcomes of Maxillary Sinus Floor Augmentation With Synthetic Bone Substitutes for Dental Implant Treatment: A Meta-Analysis.J Oral Implantol. 2022 Apr 1;48(2):158-167. doi: 10.1563/aaid-joi-D-20-00202. J Oral Implantol. 2022. PMID: 33465775
-
[Bone substitutes used for sinus lift].HNO. 2015 Jul;63(7):481-8. doi: 10.1007/s00106-015-0031-8. HNO. 2015. PMID: 26156659 Review. German.
Cited by
-
The Progress of Decellularized Scaffold in Stomatology.Tissue Eng Regen Med. 2022 Jun;19(3):451-461. doi: 10.1007/s13770-022-00432-w. Epub 2022 Mar 23. Tissue Eng Regen Med. 2022. PMID: 35320505 Free PMC article. Review.
-
Radiographic Analysis of Graft Dimensional Changes after Lateral Maxillary Sinus Augmentation with Heterologous Materials and Simultaneous Implant Placement: A Retrospective Study in 18 Patients.Materials (Basel). 2022 Apr 22;15(9):3056. doi: 10.3390/ma15093056. Materials (Basel). 2022. PMID: 35591390 Free PMC article.
-
Biomaterials for Periodontal and Peri-Implant Regeneration.Materials (Basel). 2021 Jun 15;14(12):3319. doi: 10.3390/ma14123319. Materials (Basel). 2021. PMID: 34203989 Free PMC article. Review.
-
Sinus Augmentation with Biomimetic Nanostructured Matrix: Tomographic, Radiological, Histological and Histomorphometrical Results after 6 Months in Humans.Front Physiol. 2017 Aug 3;8:565. doi: 10.3389/fphys.2017.00565. eCollection 2017. Front Physiol. 2017. PMID: 28824459 Free PMC article.
-
Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review.Materials (Basel). 2022 Jan 31;15(3):1120. doi: 10.3390/ma15031120. Materials (Basel). 2022. PMID: 35161065 Free PMC article. Review.
References
-
- Smiler D. G., Johnson P. W., Lozada J. L., et al. Sinus lift grafts and endosseous implants. Treatment of the atrophic posterior maxilla. Dental Clinics of North America. 1992;36(1):151–187. - PubMed
-
- Iezzi G., Piattelli A., Giuliani A., et al. Molecular, cellular and pharmaceutical aspects of bone grafting materials and membranes during maxillary Sinus-lift Procedures. Part 1: A General Overview. Current Pharmaceutical Biotechnology. 2017;18(1):19–32. doi: 10.2174/1389201017666161221155237. - DOI - PubMed
-
- Del Fabbro M., Testori T., Francetti L., Weinstein R. Systematic review of survival rates for implants placed in the grafted maxillary sinus. International Journal of Periodontics and Restorative Dentistry. 2004;24(6):565–577. - PubMed
-
- dos Anjos T., de Molon R., Paim P., Marcantonio E., Marcantonio E., Jr., Faeda R. Implant stability after sinus floor augmentation with deproteinized bovine bone mineral particles of different sizes: a prospective, randomized and controlled split-mouth clinical trial. International Journal of Oral and Maxillofacial Surgery. 2016;45(12):1556–1563. doi: 10.1016/j.ijom.2016.09.004. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

