Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults
- PMID: 28349517
- PMCID: PMC6464559
- DOI: 10.1002/14651858.CD012332.pub2
Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults
Abstract
Background: Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used in the treatment of pain in fibromyalgia, despite being considered not to be effective.
Objectives: To assess the analgesic efficacy, tolerability (drop-out due to adverse events), and safety (serious adverse events) of oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults.
Search methods: We searched CENTRAL, MEDLINE, and Embase for randomised controlled trials from inception to January 2017. We also searched the reference lists of retrieved studies and reviews, and online clinical trial registries.
Selection criteria: We included randomised, double-blind trials of two weeks' duration or longer, comparing any oral NSAID with placebo or another active treatment for relief of pain in fibromyalgia, with subjective pain assessment by the participant.
Data collection and analysis: Two review authors independently extracted data and assessed trial quality and potential bias. Primary outcomes were participants with substantial pain relief (at least 50% pain relief over baseline or very much improved on Patient Global Impression of Change scale (PGIC)) or moderate pain relief (at least 30% pain relief over baseline or much or very much improved on PGIC), serious adverse events, and withdrawals due to adverse events; secondary outcomes were adverse events, withdrawals due to lack of efficacy, and outcomes relating to sleep, fatigue, and quality of life. Where pooled analysis was possible, we used dichotomous data to calculate risk difference (RD) and number needed to treat for an additional beneficial outcome (NNT), using standard methods. We assessed the quality of the evidence using GRADE and created a 'Summary of findings' table.
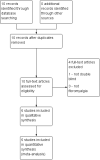
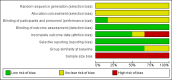
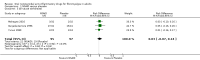
Main results: Our searches identified six randomised, double-blind studies involving 292 participants in suitably characterised fibromyalgia. The mean age of participants was between 39 and 50 years, and 89% to 100% were women. The initial pain intensity was around 7/10 on a 0 to 10 pain scale, indicating severe pain. NSAIDs tested were etoricoxib 90 mg daily, ibuprofen 2400 mg daily, naproxen 1000 mg daily, and tenoxicam 20 mg daily; 146 participants received NSAID and 146 placebo. The duration of treatment in the double-blind phase varied between three and eight weeks.Not all studies reported all the outcomes of interest. Analyses consistently showed no significant difference between NSAID and placebo: substantial benefit (at least 50% pain intensity reduction) (risk difference (RD) -0.07 (95% confidence interval (CI) -0.18 to 0.04) 2 studies, 146 participants; moderate benefit (at least 30% pain intensity reduction) (RD -0.04 (95% CI -0.16 to 0.08) 3 studies, 192 participants; withdrawals due to adverse events (RD 0.04 (95% CI -0.02 to 0.09) 4 studies, 230 participants; participants experiencing any adverse event (RD 0.08 (95% CI -0.03 to 0.19) 4 studies, 230 participants; all-cause withdrawals (RD 0.03 (95% CI -0.07 to 0.14) 3 studies, 192 participants. There were no serious adverse events or deaths. Although most studies had some measures of health-related quality of life, fibromyalgia impact, or other outcomes, none reported the outcomes beyond saying that there was no or little difference between the treatment groups.We downgraded evidence on all outcomes to very low quality, meaning that this research does not provide a reliable indication of the likely effect. The likelihood that the effect could be substantially different is very high. This is based on the small numbers of studies, participants, and events, as well as other deficiencies of reporting study quality allowing possible risks of bias.
Authors' conclusions: There is only a modest amount of very low-quality evidence about the use of NSAIDs in fibromyalgia, and that comes from small, largely inadequate studies with potential risk of bias. That bias would normally be to increase the apparent benefits of NSAIDs, but no such benefits were seen. Consequently, NSAIDs cannot be regarded as useful for treating fibromyalgia.
Conflict of interest statement
SD: none known.
PW: none known.
WH is a specialist in general internal medicine, psychosomatic medicine and pain medicine, who treats patients with fibromyalgia. He is a member of the medical board of the German Fibromyalgia Association. He is the head of the steering committee of the German guideline on fibromyalgia and a member of the steering committee of the European League Against Rheumatism (EULAR) update recommendations on the management of fibromyalgia. He received speaking fees for one educational lecture each from MSD Sharpe & Dohme (2014) and Grünenthal (2015) on pain management.
MM: none known; MM is a specialist physician who treats patients with fibromyalgia.
TRT is a site investigator for the Neuropain project, funded by Pfizer. Since 2014 TRT has consulted with or received lecture fees from pharmaceutical companies related to chronic pain and analgesics: Allergan, Boehringer, Esteve, Hexal, Jannssen‐Cilag, RB.
RB: none known; RB is a retired specialist pain physician who worked with chronic pain patients, including fibromyalgia patients.
RAM has received grant support from Grünenthal relating to individual patient‐level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Figures




Update of
References
References to studies included in this review
Goldenberg 1986 {published data only}
Kravitz 1994 {published data only}
-
- Kravitz HM, Katz RS, Helmke N, Jeffriess H, Bukovsky J, Fawcett J. Alprazolam and ibuprofen in the treatment of fibromyalgia‐report of a double‐blind placebo‐controlled study. Journal of Musculoskeletal Pain 1994;2(1):3‐27.
Mahagna 2016 {published data only}
Quijada‐Carrera 1996 {published data only}
-
- Quijada‐Carrera J, Valenzuela‐Castaño A, Povedano‐Gómez J, Fernández‐Rodriguez A, Hernánz‐Mediano W, Gutierrez‐Rubio A, et al. Comparison of tenoxicam and bromazepan in the treatment of fibromyalgia: a randomized, double‐blind, placebo‐controlled trial. Pain 1996;65(2):221‐5. - PubMed
Russell 1991 {published data only}
Yunus 1989 {published data only}
-
- Yunus MB, Masi AT, Aldag JC. Short term effects of ibuprofen in primary fibromyalgia syndrome: a double blind, placebo controlled trial. Journal of Rheumatology 1989;16(4):527‐32. - PubMed
References to studies excluded from this review
Donald 1980 {published data only}
-
- Donald JF, Layes Molla A. A comparative double‐blind study of tiaprofenic acid and aspirin in the treatment of muscular rheumatism, fibrositis, sprains and soft tissue injuries in general practice. Journal of International Medical Research 1980;8(6):382‐7. - PubMed
Fossaluzza 1992 {published data only}
-
- Fossaluzza V, Vita S. Combined therapy with cyclobenzaprine and ibuprofen in primary fibromyalgia syndrome. International Journal of Clinical Pharmacology Research 1992;12(2):99‐102. - PubMed
Le Gallez 1988 {published data only}
-
- Gallez P, Reeve FB, Crawley MA, Bird HA. A double‐blind comparison of ibuprofen, placebo and ibuprofen with meptazinol in soft tissue rheumatism. Current Medical Research and Opinion 1988;10(10):663‐7. - PubMed
Schorn 1986 {published data only}
-
- Schorn D. Tenoxicam in soft‐tissue rheumatism. South African Medical Journal 1986;69(5):301‐3. - PubMed
Additional references
Arnold 2013
Bennett 2007
Bennett 2009
Bhala 2014
-
- Coxib and traditional NSAID Trialists' (CNT) Collaboration, Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: meta‐analyses of individual participant data from randomised trials. Lancet 2014;382(9894):769‐79. [DOI: 10.1016/S0140-6736(13)60900-9] - DOI - PMC - PubMed
Bradley 2009
Calvo 2012
Choi 2010
Clauw 2014
Cohen 1988
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Abingdon, UK: Lawrence Erlbaum Associates, 1988. [ISBN: 978‐0805802832]
Conaghan 2015
-
- Conaghan PG, Peloso PM, Everett SV, Rajagopalan S, Black CM, Mavros P, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real‐world therapies. Rheumatology (Oxford) 2015;54(2):270‐7. [DOI: 10.1093/rheumatology/keu332] - DOI - PMC - PubMed
Dechartres 2013
Dechartres 2014
Dworkin 2008
Eich 2012
EMEA 2005
-
- European Medicines Agency. EMEA public statement on the suspension of the marketing authorisation for Bextra (valdecoxib) in the European Union. ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/12/WC500... (accessed 23 August 2016) 2005.
EPOC 2015
-
- Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services 2015; Vol. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 30 November 2016).
Fayers 2014
FDA 2004
-
- Food, Drug Administration. FDA Public Health Advisory: safety of Vioxx. www.fda.gov/drugs/drugSafety/postmarketdrugsafetyinformationforpatientsa... (accessed 23 August 2016) 2004.
Fitzcharles 2013
Forseth 1999
-
- Forseth KO, Husby G, Gran JT, Førre O. Prognostic factors for the development of fibromyalgia in women with self‐reported musculoskeletal pain. A prospective study. Journal of Rheumatology 1999;26:2458‐67. [PUBMED: 10555910] - PubMed
Geiss 2012
Gilron 2013
Guyatt 2011
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Guyatt 2013b
Hasnie 2007
-
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus‐associated pain: relationship between mechanical hypersensitivity and anxiety‐related behavior, and the influence of analgesic drugs. Neuroscience 2007;144(4):1495‐508. [DOI: 10.1016/j.neuroscience.2006.11.029] - DOI - PMC - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2010
Häuser 2010
Häuser 2011
Häuser 2012
Häuser 2013a
-
- Häuser W, Galek A, Erbslöh‐Möller B, Köllner V, Kühn‐Becker H, Langhorst J, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154(8):1216‐23. [DOI: 10.1016/j.pain.2013.03.034] - DOI - PubMed
Häuser 2013b
Häuser 2014a
Häuser 2014b
Häuser 2015
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] - PubMed
Kadetoff 2012
Kalso 2013
Kawakami 2002
-
- Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Tamaki T. Epidural injection of cyclooxygenase‐2 inhibitor attenuates pain‐related behavior following application of nucleus pulposus to the nerve root in the rat. Journal of Orthopaedic Research 2002;20(2):376‐81. [DOI: 10.1016/S0736-0266(01)00114-0] - DOI - PubMed
Koroschetz 2011
L'Abbé 1987
Laine 2001
Lange 2010
Lee 2012
Lunn 2014
Macfarlane 2017
Mangoni 2010
-
- Mangoni AA, Woodman RJ, Gaganis P, Gilbert AL, Knights KM. Use of non‐steroidal anti‐inflammatory drugs and risk of incident myocardial infarction and heart failure, and all‐cause mortality in the Australian veteran community. British Journal of Clinical Pharmacology 2010;69(6):689‐700. [DOI: 10.1111/j.1365-2125.2010.03627.x] - DOI - PMC - PubMed
Mansfield 2016
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐262718‐X]
Mease 2009
Mills 2015
Moher 2009
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009
Moore 2010a
Moore 2010b
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010c
Moore 2010d
Moore 2011a
Moore 2011b
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2011c
Moore 2012a
Moore 2012b
Moore 2013a
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2014c
Moore 2015
Mork 2010
-
- Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: longitudinal data from the Norwegian Nord‐Trøndelag Health Study. Arthritis Care & Research 2010;62:611‐7. - PubMed
Mork 2012
Nüesch 2013
O'Brien 2010
Oaklander 2013
PaPaS 2012
-
- Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 23 August 2016).
Peloso 2016
-
- Peloso PM, Moore RA, Chen WJ, Lin HY, Gates DF, Straus WL, et al. Osteoarthritis patients with pain improvement are highly likely to also have improved quality of life and functioning. A post hoc analysis of a clinical trial. Scandinavian Journal of Pain 2016;13(10):175‐81. [DOI: 10.1016/j.sjpain.2016.07.002] - DOI - PubMed
Queiroz 2013
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 1986
-
- Russell IJ, Vipraio GA, Morgan WW, Bowden CL. Is there a metabolic basis for the fibrositis syndrome?. American Journal of Medicine 1986;81(Suppl 3A):50‐54. - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shaver 2009
Sommer 2012a
Sommer 2012b
-
- Sommer C, Häuser W, Alten R, Petzke F, Späth M, Tölle T, et al. Drug therapy of fibromyalgia syndrome. Systematic review, meta‐analysis and guideline [Arbeitsgemeinschaft der wissenschaftlichen medizinischen fachgesellschaften]. Schmerz 2012;26(3):297‐310. [DOI: 10.1007/s00482-012-1172-2] - DOI - PubMed
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Straube 2011
Sultan 2008
Vo 2009
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
Walitt 2015
Wiffen 2013
Wolfe 1990
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33(2):160‐72. [DOI: 10.1002/art.1780330203] - DOI - PubMed
Wolfe 2010
Wolfe 2011
-
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology 2011;38:1113‐22. [DOI: 10.3899/jrheum.100594] - DOI - PubMed
Wolfe 2013
Wolfe 2014a
Wolfe 2014b
Yunus 1981
-
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Seminars in Arthritis and Rheumatism 1981;11(1):151‐71. - PubMed
Yunus 2008
Üçeyler 2013a
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

