Advanced glycation end products promote the proliferation and migration of primary rat vascular smooth muscle cells via the upregulation of BAG3
- PMID: 28350077
- PMCID: PMC5403185
- DOI: 10.3892/ijmm.2017.2938
Advanced glycation end products promote the proliferation and migration of primary rat vascular smooth muscle cells via the upregulation of BAG3
Abstract
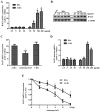
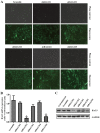
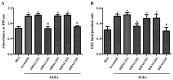
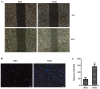
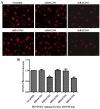
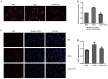
The present study was aimed to investigate the role of reactive oxygen species (ROS) on advanced glycation end product (AGE)-induced proliferation and migration of vascular smooth muscle cells (VSMCs) and whether Bcl-2‑associated athanogene 3 (BAG3) is involved in the process. Primary rat VSMCs were extracted and cultured in vitro. Cell viability was detected by MTT assay and cell proliferation was detected by EdU incorporation assay. Cell migration was detected by wound healing and Transwell assays. BAG3 was detected using qPCR and western blot analysis. Transcriptional and translational inhibitors (actinomycin D and cycloheximide, respectively) were used to study the effect of AGEs on the expression of BAG3 in VSMCs. Lentiviral plasmids containing short hairpin RNA (shRNA) against rat BAG3 or control shRNA were transduced into VSMCs. Cellular ROS were detected by 2',7'-dichlorofluorescein diacetate (DCFH-DA) staining. Mitochondrial membrane potential was detected by tetramethylrhodamine methyl ester (TMRE) staining. AGEs significantly increased the expression of BAG3 in a dose-and time-dependent manner. Furthermore, AGEs mainly increased the expression of BAG3 mRNA by increasing the RNA synthesis rather than inhibiting the RNA translation. BAG3 knockdown reduced the proliferation and migration of VSMCs induced by AGEs. BAG3 knockdown reduced the generation of ROS and sustained the mitochondrial membrane potential of VSMCs. Reduction of ROS production by N-acetylcysteine (NAC), a potent antioxidant, also reduced the proliferation and migration of VSMCs. On the whole, the present study demonstrated for the first time that AGEs could increase ROS production and promote the proliferation and migration of VSMCs by upregulating BAG3 expression. This study indicated that BAG3 should be considered as a potential target for the prevention and/or treatment of vascular complications of diabetes.
Figures




Similar articles
-
Role of advanced glycation end products on vascular smooth muscle cells under diabetic atherosclerosis.Front Endocrinol (Lausanne). 2022 Aug 31;13:983723. doi: 10.3389/fendo.2022.983723. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36120471 Free PMC article. Review.
-
BAG3 promotes the phenotypic transformation of primary rat vascular smooth muscle cells via TRAIL.Int J Mol Med. 2018 May;41(5):2917-2926. doi: 10.3892/ijmm.2018.3493. Epub 2018 Feb 14. Int J Mol Med. 2018. PMID: 29484366
-
Advanced glycation end products promote proliferation and suppress autophagy via reduction of Cathepsin D in rat vascular smooth muscle cells.Mol Cell Biochem. 2015 May;403(1-2):73-83. doi: 10.1007/s11010-015-2338-x. Epub 2015 Feb 12. Mol Cell Biochem. 2015. PMID: 25673507
-
Regulation of angiotensin II-induced B-cell lymphoma-2-associated athanogene 3 expression in vascular smooth muscle cells.Mol Med Rep. 2018 Apr;17(4):6156-6162. doi: 10.3892/mmr.2018.8630. Epub 2018 Feb 22. Mol Med Rep. 2018. PMID: 29484407
-
Reactive Oxygen Species: Modulators of Phenotypic Switch of Vascular Smooth Muscle Cells.Int J Mol Sci. 2020 Nov 20;21(22):8764. doi: 10.3390/ijms21228764. Int J Mol Sci. 2020. PMID: 33233489 Free PMC article. Review.
Cited by
-
The role of nonmyocardial cells in the development of diabetic cardiomyopathy and the protective effects of FGF21: a current understanding.Cell Commun Signal. 2024 Sep 26;22(1):446. doi: 10.1186/s12964-024-01842-0. Cell Commun Signal. 2024. PMID: 39327594 Free PMC article. Review.
-
Influences of advanced glycosylation end products on the inner blood-retinal barrier in a co-culture cell model in vitro.Open Life Sci. 2020 Aug 24;15(1):619-628. doi: 10.1515/biol-2020-0067. eCollection 2020. Open Life Sci. 2020. PMID: 33817250 Free PMC article.
-
Dietary Chrysin Suppresses Formation of Actin Cytoskeleton and Focal Adhesion in AGE-Exposed Mesangial Cells and Diabetic Kidney: Role of Autophagy.Nutrients. 2019 Jan 9;11(1):127. doi: 10.3390/nu11010127. Nutrients. 2019. PMID: 30634545 Free PMC article.
-
Role of advanced glycation end products on vascular smooth muscle cells under diabetic atherosclerosis.Front Endocrinol (Lausanne). 2022 Aug 31;13:983723. doi: 10.3389/fendo.2022.983723. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36120471 Free PMC article. Review.
-
AGE-RAGE Stress in the Pathophysiology of Pulmonary Hypertension and its Treatment.Int J Angiol. 2019 Jun;28(2):71-79. doi: 10.1055/s-0039-1687818. Epub 2019 Apr 19. Int J Angiol. 2019. PMID: 31384104 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

