TP53 and RET may serve as biomarkers of prognostic evaluation and targeted therapy in hepatocellular carcinoma
- PMID: 28350084
- PMCID: PMC5367355
- DOI: 10.3892/or.2017.5494
TP53 and RET may serve as biomarkers of prognostic evaluation and targeted therapy in hepatocellular carcinoma
Erratum in
-
[Corrigendum] TP53 and RET may serve as biomarkers of prognostic evaluation and targeted therapy in hepatocellular carcinoma.Oncol Rep. 2022 Nov;48(5):196. doi: 10.3892/or.2022.8411. Epub 2022 Sep 21. Oncol Rep. 2022. PMID: 36129137 Free PMC article.
Abstract
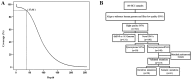
Hepatocellular carcinoma (HCC) is the most common malignancy of the liver. Genomic analysis is conducted to identify genetic alterations in driver genes which are all druggable targets for cancer therapy. In the present study, we performed an exome sequencing of 45 driver genes in 100 paired samples from HCC patients including tumors and matched adjacent normal tissues using Illumina HiSeq 2000 platform. Non-synonymous mutations were ascertained using the iPLEX MassARRAY system and Sanger sequencing. Clinicopathological relevance with genetic variations was assessed using SPSS software. The prognostic analyses of patients with gene mutation status were summarized using Kaplan-Meier curves. Sixty-one non-synonymous somatic mutations were identified in 43% of the HCC patients. The most frequent mutations were: TP53 (20%), RET (6%), PLCE1 (5%), PTEN (4%) and VEGFR2 (3%). Patients with mutations in TP53 had a lower overall survival (OS) (P=0.002) than those without mutations. Recurrent mutations in the Ret proto‑oncogene (RET) were associated with poor outcomes for both disease‑free survival (DFS) (P=0.028) and OS (P=0.001) in HCC patients. The mutational status of sorafenib-targeted genes were associated with decreased DFS (P=0.039), and decreased OS (P=0.15) without statistical significance. Mutual exclusion of TP53 and RET mutations were observed in the present study. In conclusion, patients with TP53 mutations, RET mutations and sorafenib-targeted gene mutations were demonstrated to be associated with poor HCC prognosis, which suggests that both TP53 and RET may serve as biomarkers of prognostic evaluation and targeted therapy in HCC.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

