miR‑150 inhibits proliferation and tumorigenicity via retarding G1/S phase transition in nasopharyngeal carcinoma
- PMID: 28350089
- PMCID: PMC5363880
- DOI: 10.3892/ijo.2017.3909
miR‑150 inhibits proliferation and tumorigenicity via retarding G1/S phase transition in nasopharyngeal carcinoma
Abstract
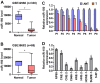
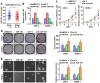
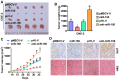
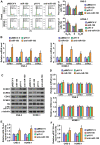
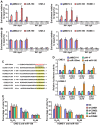
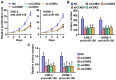
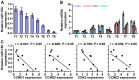
Cancer cells are characterized by a pathological manifestation of uncontrolled proliferation, which results in tumor formation. Therefore, it is necessary to improve understanding of the underlying mechanism of cell cycle control. Here, we report that miR‑150 is downregulated in nasopharyngeal carcinoma tissues and cells. Upregulation of miR‑150 suppresses nasopharyngeal carcinoma (NPC) cell proliferation and induces G1/S arrest in vitro, and inhibits tumorigenesis in vivo. Conversely, silencing miR‑150 yields the opposite effect. Our results further demonstrate that miR‑150 retards nasopharyngeal carcinoma cell proliferation and G1/S transition via targeting multiple cell cycle-related genes, including CCND1, CCND2, CDK2 and CCNE2. Therefore, our results uncover a novel mechanistic understanding of miR‑150-mediated tumor suppression in NPC, which will facilitate the development of effective cancer therapies against nasopharyngeal carcinoma.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

