Advancing Diagnostics to Address Antibacterial Resistance: The Diagnostics and Devices Committee of the Antibacterial Resistance Leadership Group
- PMID: 28350903
- PMCID: PMC5848376
- DOI: 10.1093/cid/ciw831
Advancing Diagnostics to Address Antibacterial Resistance: The Diagnostics and Devices Committee of the Antibacterial Resistance Leadership Group
Abstract
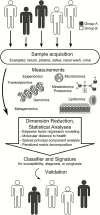
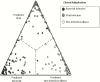
Diagnostics are a cornerstone of the practice of infectious diseases. However, various limitations frequently lead to unmet clinical needs. In most other domains, diagnostics focus on narrowly defined questions, provide readily interpretable answers, and use true gold standards for development. In contrast, infectious diseases diagnostics must contend with scores of potential pathogens, dozens of clinical syndromes, emerging pathogens, rapid evolution of existing pathogens and their associated resistance mechanisms, and the absence of gold standards in many situations. In spite of these challenges, the importance and value of diagnostics cannot be underestimated. Therefore, the Antibacterial Resistance Leadership Group has identified diagnostics as 1 of 4 major areas of emphasis. Herein, we provide an overview of that development, highlighting several examples where innovation in study design, content, and execution is advancing the field of infectious diseases diagnostics.
Keywords: antibacterial resistance; devices.; diagnostics.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Evans S, Tran T, Hujer A, et al. Choosing ceftazidime/avibactam and ceftolozane/tazobactam as empiric therapies against Pseudomonas aeruginosa (Pa) using rapid molecular diagnostics (RMDs): PRIMERS IV. IDWeek. New Orleans, LA, 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

